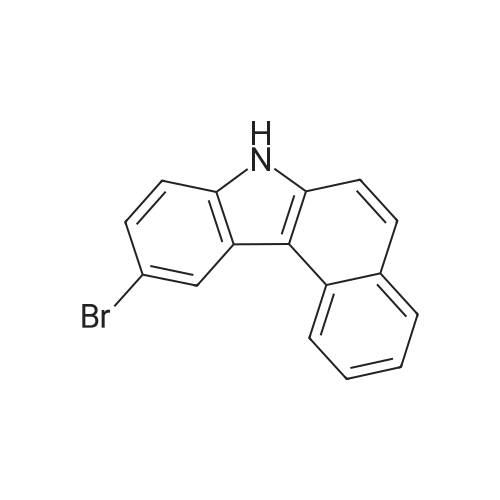
| 78% |
With N-Bromosuccinimide; In N,N-dimethyl-formamide; at 20℃; for 24h; |
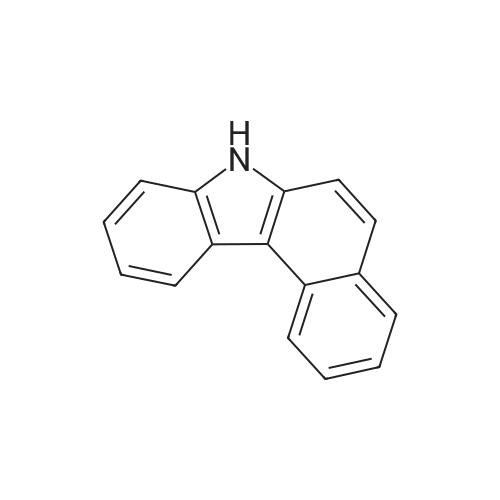
Preparation of Compound 7-1 [193] 7H-benzo[c]carbazole (50g, 0.23mol) was dissolved in DMF 1.4L, and NBS (41g, 0.23mol) was added, after which the mixture was stirred at room temperature for 24 hours. After termination of the reaction, the resultant mixture was extracted with EA and the organic layer was distilled under reduced pressure. Silica column separation was then performed, yielding Compound 7-1 (53.2g, 78%). |
| 77% |
With N-Bromosuccinimide; In N,N-dimethyl-formamide; at 20℃; for 2h;Inert atmosphere; Cooling with ice; |
Under nitrogen atmosphere, N-bromosuccinimide (12.6 g) was added to a solution of 7H-benzo [c] carbazole (15.7 g) in N, N-dimethylformamide (250 mL) under ice cooling, and the temperature was raised to room temperature And the mixture was stirred for 2 hours. The resulting reaction solution was extracted with toluene, the toluene layer was washed with saturated brine, dried with anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The obtained residue was purified by silica gel column chromatography to obtain the objective 10-bromo-7H-benzo [c] carbazole (16.2 g, yield 77%) |
| 39% |
With N-Bromosuccinimide; In N,N-dimethyl-formamide; at 20℃; for 12h; |
After introducing 7H-benzo[cjcarbazole (50 g, 230 mmol) and DMF (200 mL) into a flask, the mixture was stirred, and N-bromosuccinimide (42 g, 230 mmol) dissolved in DMF (50 mL) was added thereto. The resultant mixture was stirred at room temperature for 12 hours, and extracted with distilled water and MC. The obtained organic layer was dried with magnesium sulfate, and distilled under reduced pressure. The residue was purified by column chromatography to obtain compound A (10-bromo-7H-benzo[cjcarbazole) (1 6g, yield: 23.5%) |
| 70.8 g |
With N-Bromosuccinimide; In dichloromethane; at -5 - 0℃; for 3h; |
D: Add 65.1 g of benzo [c] carbazole and 600 ml of dichloromethane to a clean and dry 1000 mL four-neck reaction flask.Stir until dissolved, then cool to -5 , add 54g NBS in batches, after the addition is complete, hold the reaction at -5-0 for 3h,HPLC followed the completion of the benzo [c] carbazole reaction. After the reaction was completed, 300 ml of clear water was added to the reaction material.After stirring for a period of time, the layers were separated, the organic phase was removed, and the solvent was removed under reduced pressure to obtain a dark brown oil.450 ml of toluene and 3 g of activated carbon were added, and the mixture was stirred under reflux for 1 h, then filtered while hot, and the solvent was removed under reduced pressure from the filtrate.A pale yellow oil was obtained, 90 ml of ethanol was added, and after dissolving, crystallized at room temperature, suction filtration was performed,A white solid was obtained, 70.8 g was obtained by drying, HPLC: 99.27%, |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping