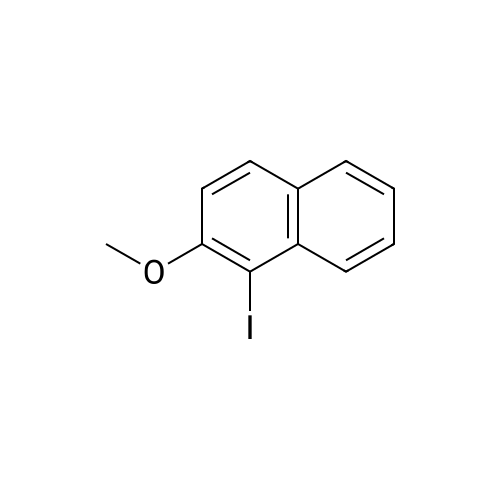
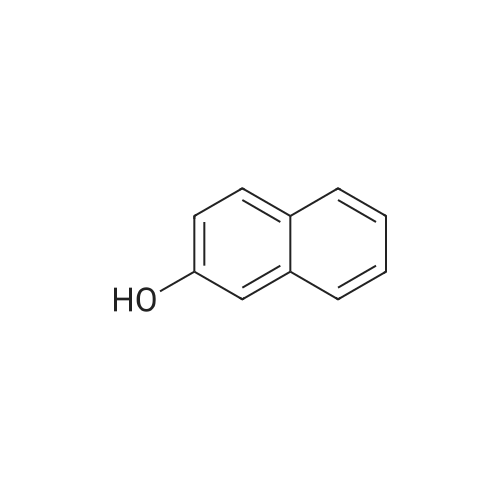
| 74% |
With sulfuric acid; dihydrogen peroxide; potassium iodide; In methanol; at 0 - 20℃; |
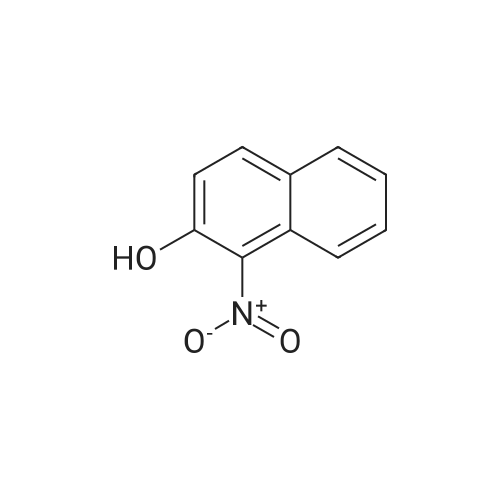
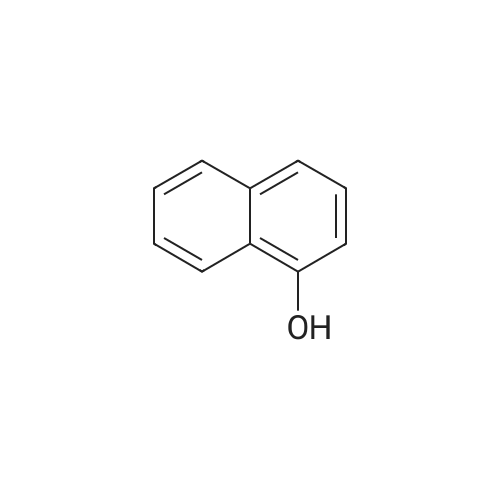
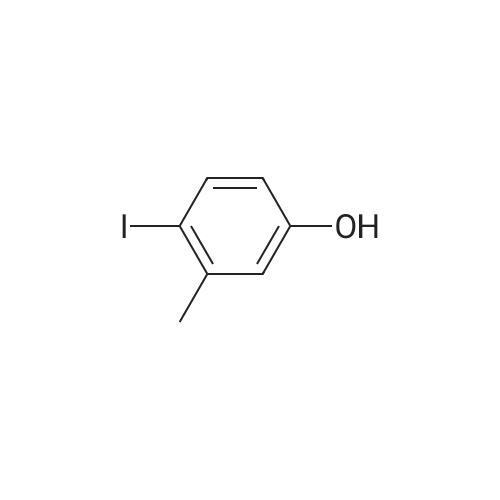
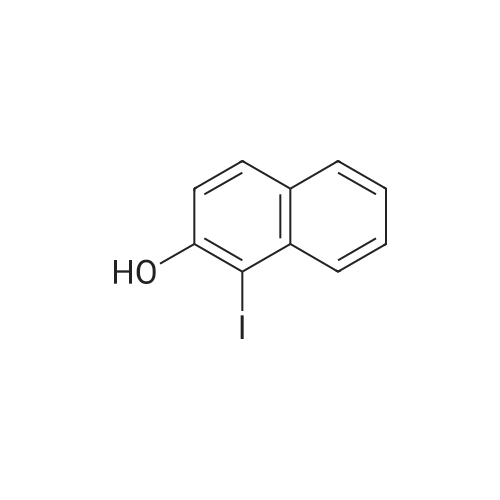
To a stirred solution of 2-naphthol (1.0g, 6.9 mmol) and conc. H2SO4 (0.54 mL 10.4 mmol) in CH3OH (20 mL) at 0 Cwas added KI (1.27 g, 7.62 mmol) and 30% H2O2 (1.56 mL, 13.9 mmol). The mixture was stirred overnight at room temperature, and then poured into CH3Cl. The mixture was washed with saturated aq. NaHSO3,with water, and dried over MgSO4, concentrated in vacuo. The crude product was purified by flash column chromatography using hexane and EtOAc to give 1-iodo-2-naphthol as light yellow solid (1.40 g, 74%), 1H NMR (300 MHz,CDCl3) d 7.91 (d, J = 8.6 Hz, 1H), 7.73-7.70 (m, 2H), 7.53 (dd, J = 7.1, 8.2 Hz, 1H), 7.36 (dd, J= 7.0, 8.1 Hz, 1H), 7.25-7.23 (m, peak merged with CDCl3, 1H), 5.79(s, 1H); 13C NMR (75 MHz, CDCl3) d 153.7, 134.7, 130.6, 130.2, 129.6, 128.3,128.1, 124.1, 116.4, 86.2; GC-MS m/z 270, 272 (M+). |
| 31% |
With sulfuric acid; dihydrogen peroxide; potassium iodide; In methanol; at 0℃; for 1h; |
This procedure has been carried out according to the following article: Synthesis 2004, No. 11, 1869 - 1873. To a solution of H2S04 (554 pL, 10.40 mmol, 1.50 equiv) in MeOH (35 mL) was added naphthalen-2-ol (1.00 g, 6.93 mmol, 1.00 equiv). The reaction was cooled at 0VC. K1 ( 1.15 g, 6.93 mmol, 1.00 equiv) and H?Oz (30% wt, 1.42 mL, 13.86 mmol, 2.00 equiv) were added. The reaction was stirred at 0C for I hour. DCM was added and the organic mixture was washed with aqueous solution of NaHS03 (0.1M), water, brine, dried over Na2S04, filtered and concentrated. The residue was purified by silica gel column chromatography (0 - 40% of DCM in Hexanes) to give the desired compound as grey solid in 31 % yield (580 mg). NMR (600 MHz, CDCb) 5 7.93 (d, /= 8.5 Hz, 1H), 7.77 - 7.72 (m, 2H), 7.55 (t, 7.4 Hz, 1H), 7.39 (t, J = 7.5 Hz, 110, 7.26 (d, 8.8 I, 1H), 5.79 (s, HD ppm. i3C NMR (151 MHz, CDCb) 5 153.86, 134.89, 130.76, 130.38, 129.78. 128.44, 128.35, 124.32, 116.57, 86.38 ppm. HRMS (ES-) calculated for CieHdD (M - H)' 268.9469, found 268.9467. IR (neat) v 3292, 1624, 1497, 1430, 1345, 1301 , 1237, 976, 924, 807, 744 cm"1. |
|
With N-iodo-succinimide;zirconium tetrachloride; In dichloromethane; at -78 - 20℃; for 2 - 3h;Conversion of starting material; |
lodination using NIS has been found to be applicable for use with a wide range of aromatic starting materials or substrates. As shown in Table 3, the iodination provides good yields and regioselectivities. In Entry 2, trace di-iodinated products were observed.Table 3 ZrCU Catalyzed lodination of Aromatic Compounds by NIS4-I : 2,4-di-l (99 : 1 )b EPO <DP n="28"/>4-1 : 2,4-di-l(36: 64)bReaction conditions: Substrate (O 5 mmol), NIS (O 5 mmol), ZrCIj (5 mol o), CH2CI2 (4 mL)" Determined by 1H NMR. 0 See spectroscopic data for characterization |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping