| 100% |
In 1,4-dioxane; for 4h;Reflux; |
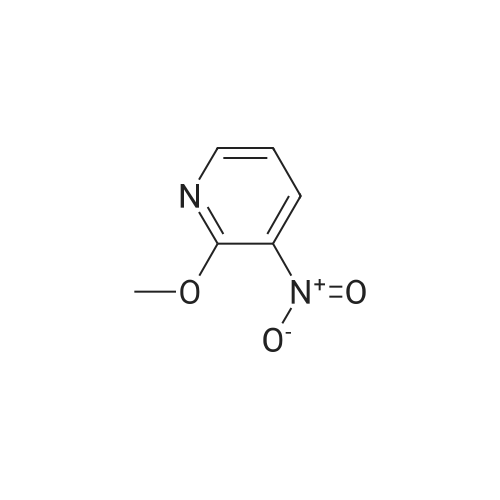
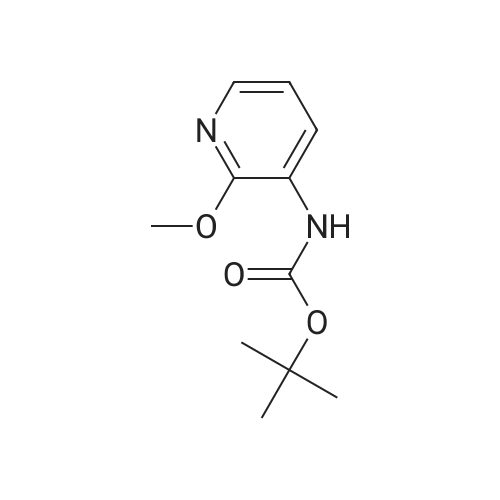
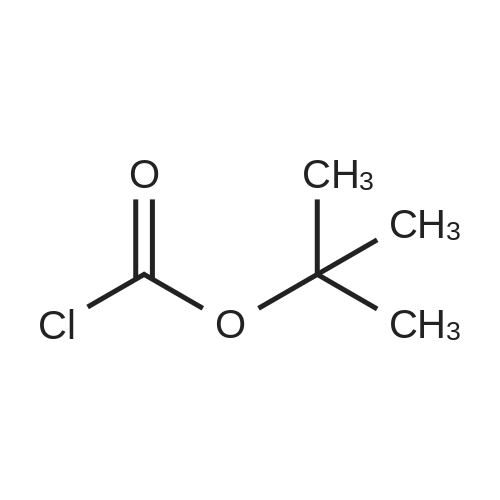
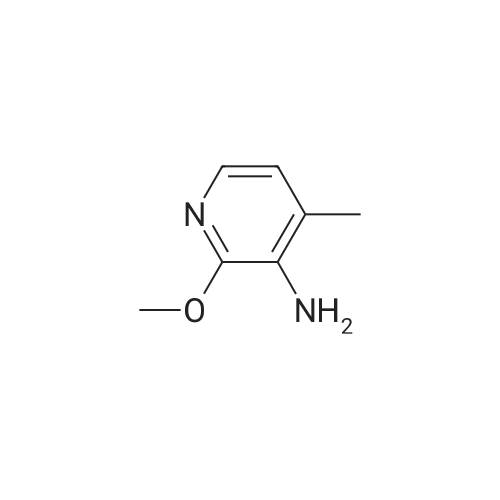
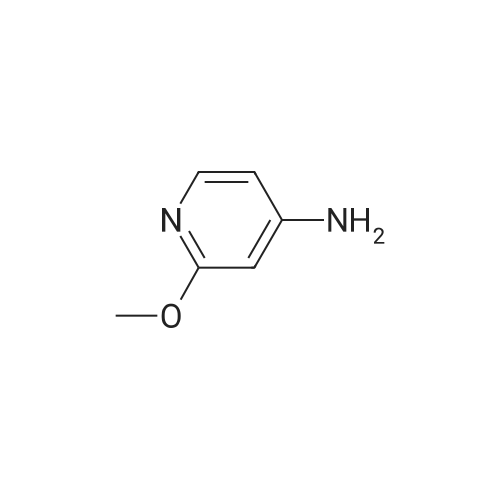
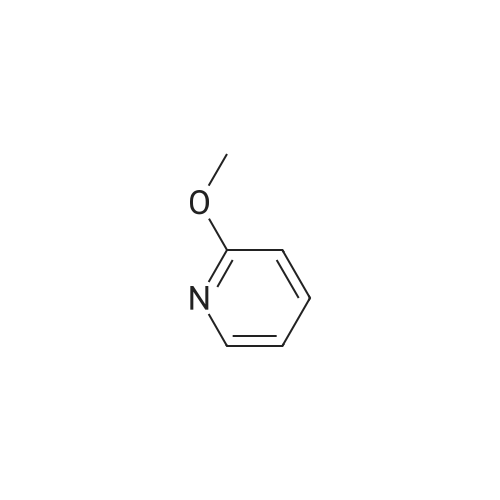
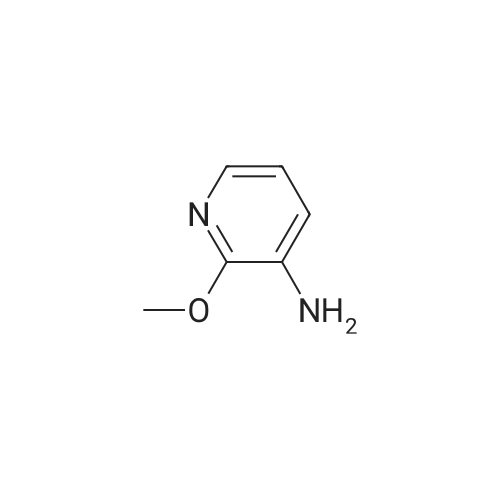
A 2000ml reaction vessel was charged with 2-methoxypyridin-3-amine 41 (100g, 0.8mol, 1wt), di-tert butyl dicarbonate (194g, 0.89mol, 1.94wt) and 1,4-dioxane (1140ml, 11.4vol). The reaction mixture was heated at reflux until complete (approx. 4 hours) as indicated by the absence of starting material by TLC analysis (eluent=1:4 ethyl acetate:hetanes, Rf starter 0.3; Rf product 0.55). The reaction mixture was concentrated to give the title compound as a dark oil (181g, 100%th.).1H NMR (400MHz, DMSO): d 1.47 (s, 9H), 3.91 (s, 3H), 6.95 (m, 1H), 7.84 (m, 1H), 7.98 (d, J=8.1Hz, 1H), 8.17 (br s, 1H). |
| 90.2% |
|
3.72 g (30 mmol) of 2-methoxy-3-pyridylamine in solution in 30 ml of tetrahydrofuran are placed in a 100-ml reactor, protected from moisture, and under a nitrogen atmosphere, and then 60 ml (60 mmol) of sodium bis(trimethylsilyl)amide in a 1 M solution in tetrahydrofuran are added dropwise at room temperature. After stirring the reaction mixture for 20 minutes at room temperature, 6.54 g (30 mmol) of di-tert-butyl carbonate are added dropwise to the reaction medium kept at room temperature. After stirring at room temperature for 3 hours, the tetrahydrofuran is evaporated off.. The residue is taken up in ethyl acetate, washed with water, with hydrochloric acid (0.1 M) and then with water (until a PH of the washings equal to 7 is obtained).. After drying the organic phase over sodium sulfate, and evaporation of the solvent, a black oil is obtained which is chromatographed on a silica gel (eluent ethyl acetate-hexane: 1-3).. After evaporation of the solvent, 6.1 g of an amber-coloured oil are obtained, that is to say a yield of 90.2%. TLC: (MERCK "Kieselgel 60" silica gel; AcOEt-hexane: 1-2); Rf=0.4 I.R.: upsilon NH=3425, CO=1731; NMR: (CDCl3): 1.5 (s, 9H); 3.95 (s, 3H); 6.8 (dd, 1H, J=5 Hz, J=7.8 Hz); 6.9 (s, 1H); 7.7 (dd, 1H, J=5 Hz, J=1.6 Hz); 8.2 (d, 1H, J=7.8 Hz). |
| 3.9 g (87%) |
With hydrogenchloride; In tetrahydrofuran; ethyl acetate; |
A) Preparation of 3-(tert-Butoxycarbonylamino)-2-methoxypyridine STR12 To a stirred solution of 2.48 g (20 mmol) of 3-amino-2-methoxypyridine and 4.37 g (20 mmol) of di-tert-butyldicarbonate (Boc2 O) in 10 mL of THF was added at 0 C. 40 mL of a 1M solution of NaHMDS in THF. The mixture was then stirred at room temperature for 2 hours. The THF was removed by rotary evaporation and the residue was dissolved in EtOAc and washed twice with an equal volume of 0.1N HCl. The EtOAc layer was dried (MgSO4) and concentrated to give 3.9 g (87%) of the desired material as an oil after purification further by flash chromatography on silica gel using 95:5 hexane:EtOAc as the eluant. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping