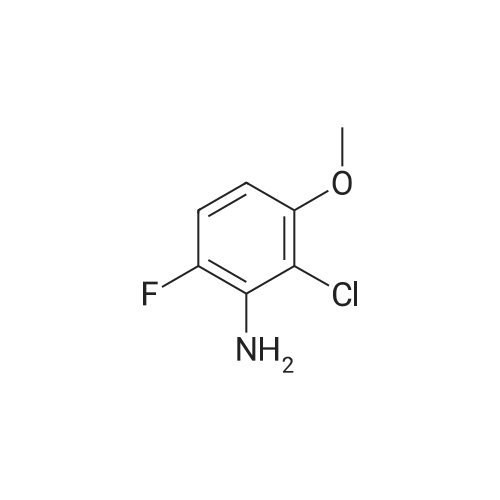
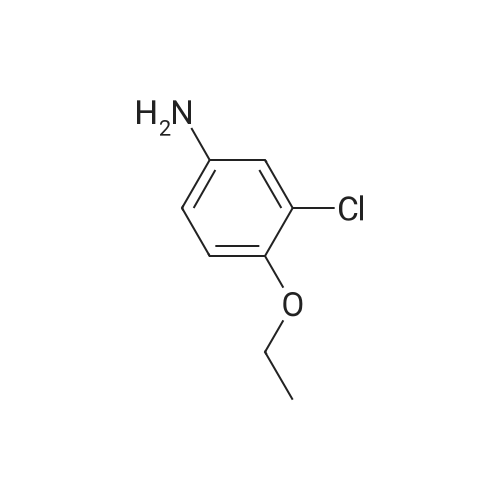
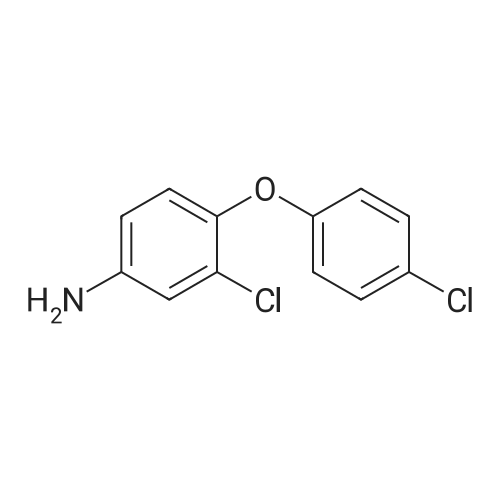
| 90 - 95% |
|
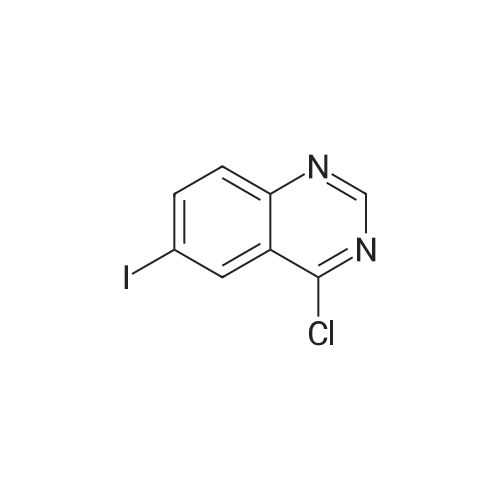
Example 1; Preparation of GW572016F; STAGE 1; A stirred suspension of 3H-6-iodoquinazolin-4-one (compound A) in toluene (5 vols) was treated with tri-n-butylamine (1.2 eq.) at 20 to 25C, then heated to 900C. Phosphorous oxychloride (1.1 eq) was added, the reaction mixture was then heated to reflux. The reaction mixture was cooled to 500C and toluene (5vols) added. Compound C (1.03 eq.) was added as a solid, the slurry was warmed back to 900C and stirred for 1 hour. The slurry was transferred to a second vessel; the first vessel was rinsed with toluene (2vol) and combined with the reaction mixture. The reaction mixture was cooled to 700C and 1.0 M aqueous sodium hydroxide solution (16 vols) added dropwise over 1 hour to the stirred slurry maintaining the contents temperature between 68-72C. The mixture was stirred at 65-700C for 1 hour and then cooled to 200C over 1 hour. The suspension was stirred at 200C for 2 hours, the product collected by filtration, and washed successively with water (3 x 5 vols) and ethanol (IMS, 2 x 5 vols), then dried in vacuo at 50-600C. <n="25"/>Volumes are quoted with respect of the quantity of Compound A used. Percent yield range observed: 90 to 95% as white or yellow crystals. |
| 90.72% |
In isopropyl alcohol;Reflux; |
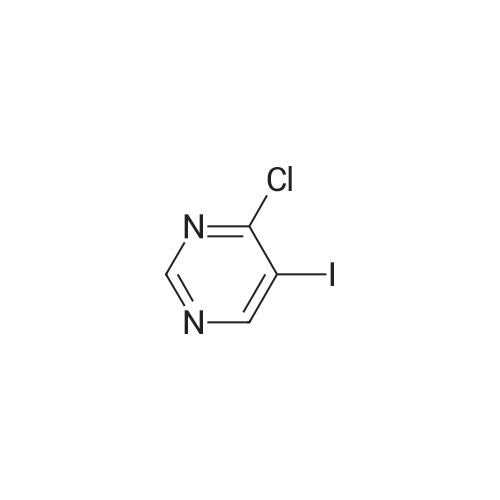
Compound B2 (5.70 g, 19.70 mmol) and compound B4 (4.90 g, 19.70 mmol) were added to a jar, while isopropanol 150 mm was added and the mixture was heated under reflux overnight. After the reaction is completed, the temperature of the reaction system is lowered to room temperature.The solid product precipitated, was decompressed and filtered to obtain a filter cake. The cake was dried to obtain 9.10 g of a product (a yield of 90.72%). The product was of high purity without purification. |
| 74.2% |
In isopropyl alcohol;Heating / reflux; |
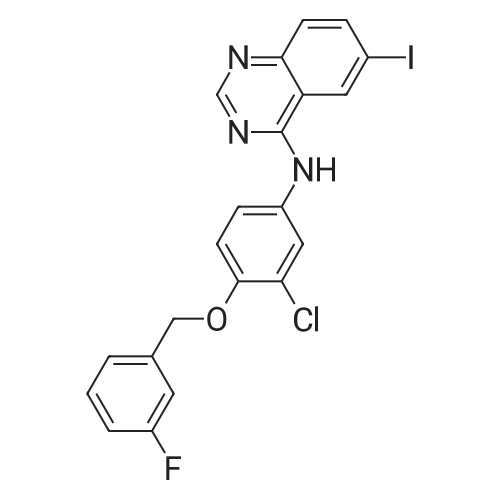
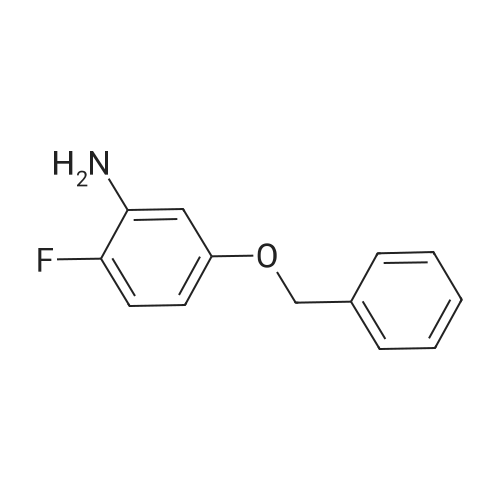
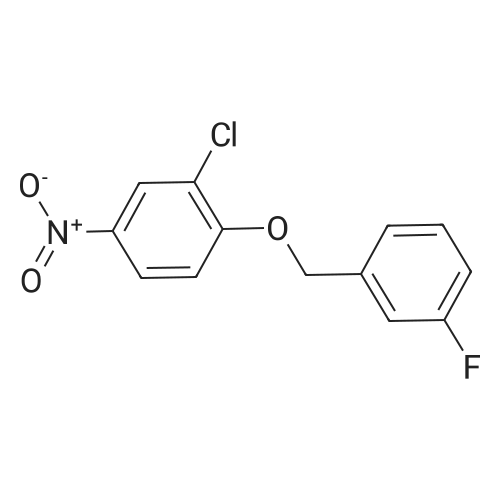
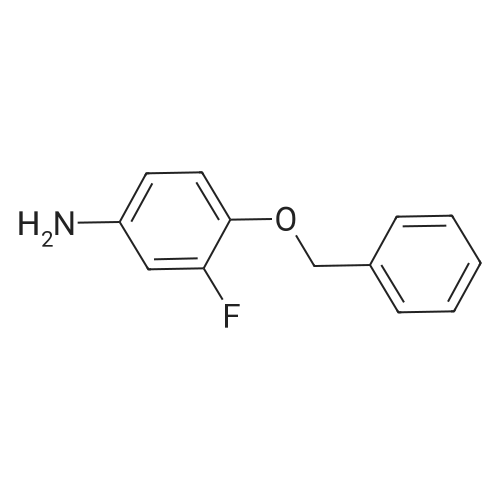
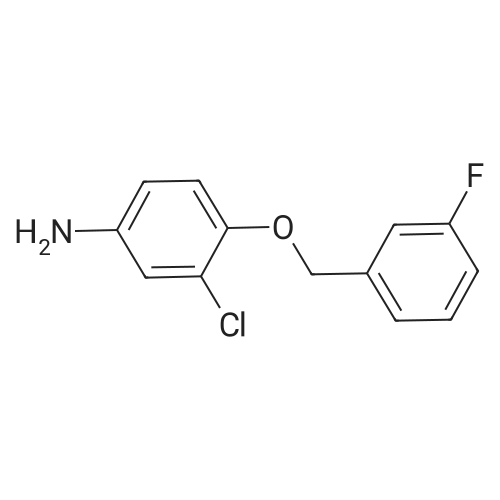
4-Chloro-6-iodoquinazoline (5.7g, 19.7 mmol) and 3-chloro-4-(3- fluorobenzyloxy)aniline (4.9g, 19.7 mmol) was refluxed in isopropanol (15OmL) overnight. The mixture was cooled to room temperature. The solid product was precipitated, filtrated and dried in vacuum. The product 1405-174 was pure enough and used without further purifcation.(7.4 g, 74.2%): LC-MS: 506 [M+l]+, 1U NMR (DMSO-J6): δ 5.29 (s, 2 H), 7.18 (m, IH), 7.33 (m, 3H), 7.48 (m, IH), 7.66 (m, IH), 7.74 (d, J= 9.0 Hz, 1 H), 7.90 (d, J= 2.2 Hz, 1 H), 8.37 (d, J= 9.0 Hz, 1 H), 8.94 (s, 1 H), 9.29 (s, 1 H). |
|
|
Roschangar et al. ,Use of lithium N,O-dimethylhydroxylamide as an efficient in situ protecting agent for aromatic aldehydes. Tertrahedron 2002, 58, 1657-1666). Preparation analogous (Nishino et al.; Process for producing 4-aminoquinazoline compound by chlorination of quinazolin-4-one or its derivative and animation. 2003) as follows: To a mixture of 6-iodo-lH-quinazolin-4-one (10) (6.8Og; 25.0 mmol), toluene (5.0 mL) and POCl3 (27.5 mmol; 2.60 mL) carefully triethylamine (27.5 mmol; 3.81 mL) was added. The mixture was heated to 80 0C for 2 h, cooled to room temperature, a solution of 3-chloro~4-(3- fluorobenzyloxy)phenylamine (15) (27.50 mmol; 6.92 g) in 2~butanone (20.0 mL) added and the mixture stirred at 80 0C for another hour. The mixture was cooled to 00C, the yellow precipitate was filtered off and added to a NaOH solution (IN; 150 mL) by stirring. After 30 min the yellow solid was filtered off, washed with water and a small amount of acetone and dried in vacuo. Yield (8.38 g; 66%) analytical pure sample. 1H-NMR (DMSO-[D6]): δ (ppm) = 5.26 (s, 2H), 7.15-7.22 (m, IH), 7.27 (d, IH, J = 9.1 Hz), 7.29-7.35 (m, 2H), 7.43-7.51 (m, IH), 7.56 (d, IH, J = 8.8 Hz), 7.74 (dd, IH, J = 9.1 Hz, 4J = 2.5 Hz), 8.02 (d, IH5 4J = 2.5 Hz), 8.12 (dd; IH, J = 8.8 Hz, 4J = 1.7 Hz)5 8.62 (s, IH), 8.96 (d, 4J = 1.7 Hz), 9.90 (s, IH, exchangeable). |
|
|
4-Chloro-6-iodoquinazoline (1wt) was added to a solution of fluorobenzyloxyaniline (0.894wt, 1.03equiv) in N-methylpyrrolidinone (8.26wt, 8vol) at ca 20C, and after the initial exotherm had subsided, the resulting solution was stirred at 20-25C for at least 30 minutes. The dark solution was treated with triethylamine (0. 58vol, 1.2equiv) and the mixture was stirred for 20-30 minutes. Isopropanol (2. 5vol) was added and the mixture was heated to ca 50C. Water (up to 3vol) was added slowly to the vessel over 10-15 minutes, while keeping the temperature at ca 50C. Once crystallisation had commenced the addition was stopped and the resulting slurry was aged for 30-45 minutes at ca 50C. Any residual water (from the 3vol) was added, then further water (5vol) was added to the vessel over ca 30 minutes while maintaining the temperature at ca 50C. The resulting slurry was cooled to ca 20C over ca 30 minutes and aged at ca 20C for at least 30 minutes. The solid was collected by filtration and washed sequentially with water (2 x 5vol), then isopropanol (5vol). The product was dried in vacuo at ca 60C to give the title compound as a cream crystalline solid. |
|
at 90℃; for 1h; |
A stirred suspension of 3H-6-iodoquinazolin-4-one (compound A) in toluene (5 vols) was treated with tri-n-butylamine (1.2 eq.) at 20 to 25C, then heated to 900C. Phosphorous oxychloride (1.1eq) was added, the reaction mixture was then heated to reflux. The reaction mixture was cooled to 500C and toluene (5vols) added. Compound C (1.03 eq.) was added as a solid, the slurry was warmed back to 900C and stirred for 1 hour. The slurry was transferred to a second vessel; the first vessel was rinsed with toluene (2vol) and combined with the reaction mixture. The reaction mixture was cooled to 7O0C and 1.0 M aqueous sodium hydroxide solution (16 vols) added dropwise over 1 hour to the stirred slurry maintaining the contents temperature between 68-72C. The mixture was stirred at 65-700C for 1 hour and then cooled to 200C over 1 hour. The suspension was stirred at 200C for 2 hours, the product collected by filtration, and washed successively with water (3 x 5 vols) and ethanol (IMS, 2 x 5 vols), then dried in vacuo at 50-600C. Volumes are quoted with respect of the quantity of Compound A used. Percent yield range observed: 90 to 95% as white or yellow crystals. |
|
|
A stirred suspension of 3W-6-iodoquinazolin-4-one in toluene (5 vols) is treated with tri-n-butylamine (1.2 equiv.), and then heated to 70-800C. Phosphorous oxychloride (1.1 equiv.) is added and the reaction mixture is then heated to reflux and stirred at this temperature for at least 2 hours. The reaction mixture is then cooled to 55C and toluene (5vol) added followed by 3-chloro-4-[(3-fluorophenyl)rnethyl]oxy}aniline (1.03 equiv.). The reaction mixture is then warmed to 70-900C and stirred for at least 2 hours. The resultant slurry is transferred to a second vessel. The temperature is adjusted to 70-750C and 8 molar aqueous sodium hydroxide solution (2 vols) added over 1 hour, followed by water (6vol.) maintaining the contents at 70-850C. The mixture is stirred at 70-850C for ca. 1 hour and then cooled to 20-250C. The suspension is stirred for ca. 2 hours and the product collected by filtration, and washed successively with water, 0.1 molar aqueous sodium hydroxide, water, and IMS, then dried in vacuo. EPO <DP n="48"/> |
|
|
A stirred suspension of 3H-6-iodoquinazolin-4-one (compound A) in toluene (5 vols) was treated with tri-n-butylamine (1.2 eq. ) at 20 to 25C, then heated to 90C. Phosphorous oxychloride (1.1eq) was added, the reaction mixture was then heated to reflux. The reaction mixture was cooled to 50C and toluene (5vols) added. Compound C (1.03 eq. ) was added as a solid, the slurry was warmed back to 90C and stirred for 1 hour. The slurry was transferred to a second vessel; the first vessel was rinsed with toluene (2vol) and combined with the reaction mixture. The reaction mixture was cooled to 70C and 1.0 M aqueous sodium hydroxide solution (16 vols) added dropwise over 1 hour to the stirred slurry maintaining the contents temperature between 68-72C. The mixture was stirred at 65-70C for 1 hour and then cooled to 20C over 1 hour. The suspension was stirred at 20C for 2 hours, the product collected by filtration, and washed successively with water (3 x 5 vols) and ethanol (IMS, 2 x 5 vols), then dried in vacuo at 50-60C. Volumes are quoted with respect of the quantity of Compound A used. Percent yield range observed: 90 to 95% as white or yellow crystals. |
|
|
The reaction mixture was cooled to 50C and toluene (5vols) added. Compound C (1.03 eq. ) was added as a solid and the slurry was warmed back to 90C and stirred for 1 hour. The slurry was transferred to a second vessel; the first vessel was rinsed with toluene (2vol) and combined with the reaction mixture. The reaction mixture was cooled to 70C and 1.0 M aqueous sodium hydroxide solution (16 vols) added dropwise over 1 hour to the stirred slurry maintaining the contents temperature between 68-72C. The mixture was stirred at 65-70C for 1 hour and then cooled to 20C over 1 hour. The suspension was stirred at 20C for 2 hours, the product collected by filtration, and washed successively with water (3 x 5 vols) and ethanol (IMS, 2 x 5 vols), then dried in vacuo at 50-60C. Volumes are quoted with respect of the quantity of Compound A used. Percent yield range observed: 90 to 95% as white or yellow crystals. |
|
|
4-Chloro-6-iodoquinazoline (1wt) was added to a solution of fluorobenzyloxyaniline (0.894wt, 1.03equiv) in N-methylpyrrolidinone (8.26wt, 8vol) at ca 200C, and after the initial exotherm had subsided, the resulting solution was stirred at 20-25C for at least 30 minutes. The dark solution was treated with triethylamine (0.58vol, 1.2equiv) and the mixture was stirred for 20-30 minutes. lsopropanol (2.5vol) was added and the mixture was heated to ca 500C. Water (up to 3vol) was added slowly to the vessel over 10-15 minutes, while keeping the temperature at ca 500C. Once crystallisation had commenced the addition was stopped and the resulting slurry was aged for 30-45 minutes at ca 500C. Any residual water (from the 3vol) was added, then further water (5vol) was added to the vessel over ca 30 minutes while maintaining the temperature at ca 500C. The resulting slurry was cooled to ca 200C over ca 30 minutes and aged at ca 200C for at least 30 minutes. The solid was collected by filtration and washed sequentially with <n="43"/>water (2 x 5vol), then isopropanol (5vol). The product was dried in vacuo at ca 600C to give the title compound as a cream crystalline solid. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping