| 87% |
With propionic acid; at 132℃; for 2h;Heating / reflux;Product distribution / selectivity; |
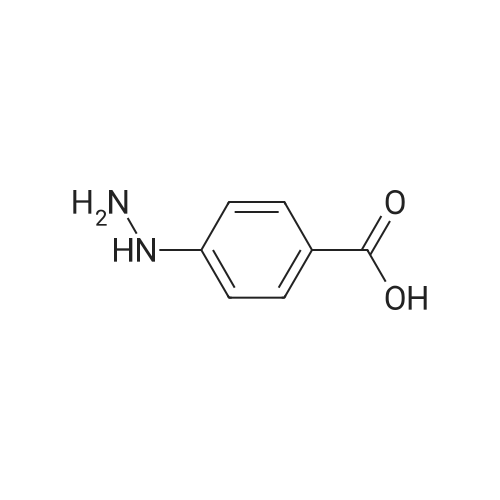
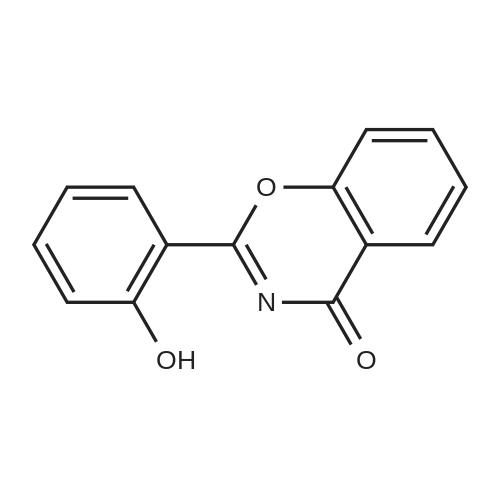
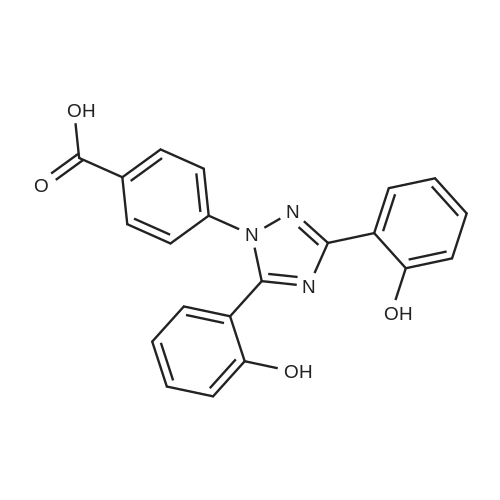
A suspension of 10.65 g (44.5 mmol) of 2-(2-hydroxyphenyl)benz[e][l,3]oxazin-4- one, 7.97 g (52.4 mmol) of 4-hydrazinobenzoic acid and 57.0 ml of propionic acid is heated to the boiling temperature of the reaction mixture and is kept at this temperature (132 0C) for 2 hours. After completion of the reaction, 57 ml of ethyl acetate is added to the suspension after cooling and the suspension is stirred for 30 minutes.The resulting crystalline product is filtered, washed with 30 ml of ethyl acetate on the filter and dried to a constant weight. A white crystalline product weighing 14.38 g is obtained, i.e. 87% of the theory, with HPLC purity above 99.4% and a melting temperature of 260 to 265 0C.Precipitation of the raw product is performed in such a way that the raw product is dissolved in a sodium hydroxide solution (6.16 g in 60 ml of water) and extraction with 50 ml of ethyl acetate is carried out. After separation the aqueous layer is filtered with 0.5 g of active carbon and after filtration the solution is acidified with hydrochloric acid to pH = 1 to 2 and the resulting suspension is stirred at the temperature of 20 0C for 30 minutes. The precipitated product is aspirated and washed with water until neutral pH. After drying to a constant weight, 14.3 g are obtained, i.e. 86% of the theory, with an HPLC purity above 99.8% and melting temperature of 263 to 265 0C.The precipitated product is stirred up with ethyl acetate by stirring in a suspension with 57 ml of ethyl acetate at a temperature of 20 0C for 30 minutes. The stirred-up product is aspirated and washed with ethyl acetate. After drying to a constant weight, 13.56 g are obtained, i.e. 82% of the theory, with HPLC purity above 99.8%. Melting temperature: 264 to 266 0C. |
| 87% |
With propionic acid; In toluene; at 110℃; for 2h;Heating / reflux;Product distribution / selectivity; |
A suspension of 0.7 g (2.93 mmol) of 2-(2-hydroxyphenyl)benz[e][l,3]oxazin-4- one and 0.52 g (3.44 mmol) of 4-liydrazinobenzoic acid in 8.0 ml of toluene and 5 ml of propionic acid is heated to its boiling point and is maintained at this temperature (110 0C) for 2 hours. After completion of the reaction, the reaction mixture is cooled and filtration is carried out. The crystalline product is washed with toluene and dried until dry.0.95 g of white product is obtained (87% of the theory), which contains 97.1% of deferasirox having a melting temperature of 256 to 263 0C.Precipitation of the raw product is performed in such a way that raw deferasirox is dissolved in a solution of 0.6 g of sodium hydroxide in 30.0 ml of water and the solution is extracted with ethyl acetate (1 x 30.0 ml). The aqueous phase is filtered with active carbon and the filtrate is acidified with concentrated hydrochloric acid until pH = 1 to 2. The suspension is stirred at the temperature of 20 0C for 1 hour and the precipitated product is filtered and washed with water. After drying, 0.73 g of precipitated deferasirox (67% of the theory) is obtained with a purity of 98.5 % and a melting temperature of 260 to 264 0C. |
| 86% |
In ethanol; for 3h;Reflux; |
A solution of compound 4 (2.39 g, 10 mmol) and 4-hydrazinobenzoic acid (1.67g,11 mmol) was heated with stirring in 30 mL ethanol for 3 h at reflux. The reaction mixture was then cooled to room temperature; the precipitated solid was collected and dried under vacuum overnight. Recrystallization and decolorization with activated charcoal in ethanol afforded 3.21 g (86% yield) of deferasirox (6) as a white solid. All spectroscopic data of product 6 matched those of an authentic sample. |
| 84% |
With propionic acid; In ethyl acetate; at 92℃; for 2h;Heating / reflux;Product distribution / selectivity; |
A suspension of 0.7 g (2.92 mmol) of 2-(24iydroxyphenyl)benz[e][l,3]oxazin-4- one and 0.52 g (3.44 mmol) of 4-hydrazinobenzoic acid in 8 ml ethyl acetate and 5.0 ml of propionic acid is heated to its boiling point and maintained at this temperature (92 0C) for 2 hours. After completion of the reaction, the mixture is cooled and filtered. The product is washed with ethyl acetate. After drying, 0.92 g of raw deferasirox is obtained, i.e. 84% of the theory, with an HPLC purity of 99.7% and a melting temperature of 258 to 263 0C. |
| 82.5% |
In ethanol;Heating / reflux;Product distribution / selectivity; |
2-(2-hydroxyphenyl)benz(e)[l,3]oxazin-4-one (15.0 g) and 4-hydrazino- benzoic acid (10.5 g) are boiled under reflux in ethanol (225 ml). The reaction is checked for completion after 2 hours by Thin Layer Chromatography (TLC). If the reaction is not complete, the reaction mixture is stirred for an additional hour and the conversion is checked again until it is complete. If the reaction is complete, the mixture is cooled to room temperature and the precipitated solid material is filtered off, washed with ethanol and dried in vacuum. Yield: 82.5 %. |
| 81% |
With propionic acid; In N,N-dimethyl-formamide; at 150℃; for 1.5h;Product distribution / selectivity; |
A suspension of 0.7 g (2.92 mmol) of 2-(2-hydroxyphenyl)benz[e][l,3]oxazin-4- one and 0.52 g (3.44 mmol) of 4-hydrazinobenzoic acid in 5 ml of N5N- dimethylformamide and 5 ml of propionic acid is heated up to 150 0C. The mixture is maintained at this temperature and stirred for 1.5 hours. After completion of the reaction, the mixture is cooled and poured onto crushed ice. The resulting crystals are aspirated and washed with water. After drying, 0.88 g of raw deferasirox is obtained, i.e. 81% of the theory, with an HPLC purity of 93% and a melting temperature of 255 to 262 0C. |
| 80% |
With triethylamine; In ethanol; for 2h;Reflux; |
2-(2-Hydroxyphenyl)-4H-3,1-benzoxazin-4-one was prepared according to the previous reported procedure with a little modifications (Lattmann and Acklin, 1997; Ryabukhin et al., 1983). 4-Hydrazino-benzoic acid (11.5mmol, 1.75g) and Et3N (11.5mmol, 1.16g) were dissolved in boiling EtOH (80ml). Then, 2-(2-Hydroxyphenyl)-4H-1,3-benzoxazin-4-one (10.45mmol, 2.50g) was added to the clear solution, and the reaction mixture was refluxed for an additional 2h. After the completion of the reaction, the solution was cooled to room temperature, and water was added until the first sign of precipitation was observed. The mixture was concentrated to a total volume of 50% under reduced pressure and aqueous 6M HCl (40ml) was added. The resulting solid was filtered, washed with water and dried for 24h in vacuo (3.11g, Yield =80%). (0011) IR (KBr, cm-1) nu 33,317, 2540, 1680 (nuC=O), 1607, 1517, 1495, 1431, 1351, 1221, 988, 752. 1H NMR (CD3COCD3, ppm): delta=7.00 (s, 1H), 7.01-7.04 (m, 3H), 7.39 (m, 2H), 7.48 (d, 1H), 7.53 (d, 2H), 8.15 (d, 2H), 8.19 (d, 1H) 10.00 (s, OH), 10.78 (s, OH) ppm. 13C NMR (CD3COCD3, ppm) delta=113.7, 113.9, 116.6 (CH), 117.0 (CH), 119.5 (CH), 119.8 (CH), 124.0 (2 CH), 126.9 (CH), 130.4 (2 CH), 130.5, 130.7 (CH), 131.4 (CH), 132.6, 141.9 (CH), 152.1, 155.6, 156.4, 160.4, 165.7 (C=O) ppm. MS (m/z) for C21H15N3O4=(374) Anal. Calc. for C21H15N3O4: C 67.56, H 4.05, N 11.25; found C 67.16, H 4.09, N 10.86. |
| 79% |
With methanol; In dichloromethane; at 0 - 70℃; for 7h; |
Example 2: Preparation of Deferasirox[0018] A mixture of methanol (450.0 ml), 2-(2-hydroxyphenyl)-benz[l ,3]oxazin- 4-one (30.0gm) were stir for 10 min at 25- 30C. To the above contents 4- hydrazino benzoic acid (20.03gm) was added. The contents were heated to reflux temperature 65-70C. The contents were maintained for 4 hours at 65-70C. The reaction mass was cooled slowly to 0-5C and maintained it for 1 hour at 0-5C. The reaction mass was filtered and washed with methanol (30.0 ml). Compound was taken into methylene chloride and stir for 10 min 25 -30C. The contents were heated to reflux temperature (40-45C) and maintained the contents for 1 hr at reflux temperature. Cool the contents to .25- 30C and stirred for lhr at 25 - 30C. The reaction mass was filtered and washed with methylene chloride (30.0ml). Dried the compound at 60-65C. Yield: 79.0%. |
| ~ 77% |
|
The moist product obtained at the end of example 2 is placed in a reactor, together with 4.88 kg of 4-hydrazinobenzoic acid and 48 kg of a 3 wt.% toluene/ethanol mixture. The mass is heated under reflux (approximately 77 C) for 2 hours. To the mass maintained with heating under reflux, 12 kg of DMF is then added, and heating under reflux is continued (at a temperature of approximately 80 C) until complete dissolution of the components present in the mixture is observed. 400 g of pharmaceutical quality decolourising carbon is then added. The suspension is heated under reflux (approximately 80 C) with stirring for 30 minutes, then it is cooled to 60 C and filtered. The filtered solution is placed in a reactor, heated under reflux, and 800 g of 85 wt.% of phosphoric acid, and 24 kg of distilled water are added. A precipitate is formed, which is heated under reflux (approximately 80 C) with continuous stirring for 30 minutes, after which the mixture is cooled to 30 C, centrifuged, and the solid is washed with a mixture consisting of 24 kg of distilled water and 8 kg of 3% toluene/ethanol.9.6 kg of raw deferasirox, dry equivalent, is obtained, determined by a weight loss test on step of the product, for a yield equal to approximately 77% in this step. The product is analysed by HPLC, determining a purity of more than 99.8%. By HPLC analysis it is determined that the 4-hydrazinobenzoic acid content of the product is below 0.5 ppm (which represents the limit of detection, LOD, of the analytical method .). |
| 60% |
With methoxypropionic acid; at 100℃; for 1h;Product distribution / selectivity; |
A suspension of 0.7 g (2.92 mmol) of 2-(2-hydroxyphenyl)benz[e][l,3]oxazin-4- one and 0.52 g (3.44 mmol) of 4-hydrazinobenzoic acid in 5 ml of methoxypropionic acid is heated to 100 0C. The mixture is maintained and stirred at this temperature for 1 hour. After completion of the reaction, the mixture is cooled and 5 ml of ethyl acetate are added. The resulting mixture is stirred for 10 minutes, then it is filtered and the crystals are washed with ethyl acetate. After drying, 0.66 g of raw deferasirox is obtained, i.e. 60% of the theory, with an HPLC content of 99.3% and a melting temperature of 259 to 263 0C. |
| 55% |
With trichloroacetic acid; In toluene; at 110℃; for 3h;Product distribution / selectivity; |
A suspension of 0.7 g (2.92 mmol) of 2-(2-hydroxyphenyl)benz[e][l,3]oxazin-4- one and 0.52 g (3.44 mmol) of 4-hydrazinobenzoic acid in 5 g of trichloroacetic acid and 5 ml of toluene is heated to 110 0C. The mixture is maintained and stirred at this temperature for 3 hours. After completion of the reaction, the mixture is cooled and poured onto crushed ice. The resulting crystals are aspirated and washed with water and ethyl acetate. After drying, 0.6 g of raw deferasirox is obtained, i.e. 55% of the theory, with an HPLC content of 99.56% and a melting temperature of 258 to 262 0C. |
| 50% |
With propionic acid; In 2-methyltetrahydrofuran; at 98℃; for 2h;Heating / reflux;Product distribution / selectivity; |
A suspension of 0.7 g (2.92 mmol) of 2-(2-hydroxyphenyl)benz[e][l,3]oxazin-4- one and 0.52 g (3.44 mmol) of 4-hydrazinobenzoic acid in 8 ml of 2- methyltetrahydrofuran and 5.0 ml of propionic acid is heated to its boiling point and maintained at this temperature (98 0C) for 2 hours. After completion of the reaction, the mixture is cooled and filtered. The product is washed with 2-methyltetrahydrofuran. After drying, 0.55 g of raw deferasirox is obtained, i.e. <n="11"/>50% of the theory, with an HPLC purity of 95.3% and a melting temperature of 255 to 262 0C. |
| 49% |
With LACTIC ACID; at 115℃; for 2h;Heating / reflux;Product distribution / selectivity; |
A suspension of 0.7 g (2.92 mmol) of 2-(2-hydroxyphenyl)benz[e][l,3]oxazin-4- one and 0.52 g (3.44 mmol) of 4-hydrazinobenzoic acid in 5 ml of lactic acid is heated to its boiling point and maintained at this temperature (115 0C) for 2 hours. After completion of the reaction the mixture is cooled down, causing separation of the crystalline product. 5 ml of ethyl acetate are added. After drying, 0.53 g of raw deferasirox is obtained, i.e. 49% of the theory, with an HPLC content of 94% and a melting temperature of 248 to 260 0C.Precipitation of the raw product is performed in such a way that raw deferasirox is dissolved in a solution of 0.23 g of sodium hydroxide in 20.0 ml of water and the solution is extracted with ethyl acetate (1 x 20.0 ml). The aqueous phase is filtered with active carbon and the filtrate is acidified with 0.75 ml of concentrated hydrochloric acid until pH = 1 to 2. The suspension is stirred at a temperature of 20 0C for 1 hour and the precipitated product is filtered and washed with water. After <n="12"/>drying, 0.31 g of precipitated deferasirox is obtained, i.e. 29% of the theory, with a purity of 98.5% and a melting temperature of 261 to 2640C.The precipitated product is stirred up in ethyl acetate by stirring in a suspension with 10 ml of ethyl acetate at a temperature of 20 0C for 30 minutes. The stirred-up product is aspirated and washed with ethyl acetate. After drying to a constant weight, 0.19 g is obtained, i.e. 17% of the theory, with a purity above 99.0%. Melting temperature: 258 to 263 0C. |
|
In ethanol; |
EXAMPLE 5 4-[3,5-Bis(2-hydroxyphenyl)-[1,2,4]triazol-1-yl]benzoic acid 5.0 g of <strong>[1218-69-5]2-(2-hydroxyphenyl)benz[e][1,3]oxazin-4-one</strong> and 3.5 g of 4-hydrazinobenzoic acid are boiled under reflux for 2 h in 75 ml of ethanol. The crystals precipitating on cooling are washed with ethanol. After drying, 4-[3,5-bis(2-hydroxyphenyl)-[1,2,4]triazol-1-yl]-benzoic acid remains as colorless crystals of m.p. 264-265 C. |
|
In methanol; for 2h;Reflux; |
2-(2-Hydroxyphenyl)-4H-l,3-benzoxazin-4-one (25g, 0.1045 mol), 4-hydrazinobenzoic acid (17.5 g, 0.115 mol) and methanol (375 ml) were added to a round bottom flask. The reaction mixture was heated to reflux temperature and refluxed for 2 hours. The resulting mass was cooled to 25C and then filtered to isolate the solid product. The wet material was added to methanol (1625 ml) and heated to 65 to 660C to provide a clear solution. The resulting solution mass was cooled to 25C, and the separated solid was filtered and then dried to produce 28.5 g of crude deferasirox (etaPLC Purity: 99.8%, content of 4-hydrazinobenzoic acid impurity: 60 ppm). |
|
|
0.1945 moles of 2-(2-Hydroxy phenyl) benz[e] [1, 3] oxazin-4-one was dissolved in 750 ml of methanol and 0.2305 moles of 4-Hydrazino benzoic acid was added at 25-300C. Subsequently, the temperature of the reaction mixture was raised to 65-700C and maintained for 3-4 hours at the same temperature. The reaction mass was cooled to 25-300C followed by 0-50C and maintained for 60 minutes. The reaction mass was then filtered and washed with chilled 100 ml of methanol.175 ml of dimethyl formamide was added to the resultant solid and stirred for 15-20 minutes to get a clear solution. The above DMF solution was slowly added to a mixture of 700 ml D M water and 14 ml concentrated HCl in 60 minutes at 25-35C and maintained for 60-90 minutes at the same temperature. The mixture was the filtered and washed with water (4 X 140 ml), dried under vacuum at 50-600C for 4-6 hours to produce 70 gm of crude 4-(3, 5- bis (2-hydroxyphenyl)-lH-l, 2, 4-triazol-l- yl) benzoic acid.The resultant solid obtained from methanol was suspended in water and heated to 50-100 C, cooled and filtered to give 4-(3, 5- bis (2-hydroxyphenyl)-lH-l, 2, 4-triazol-l-yl) benzoic acid, free from sulfated ash. |
|
In methanol; at 60 - 65℃; for 5h;Product distribution / selectivity; |
4-hydrazinobenzoic acid (38 grams) was added to the mixture of 2-(2-hydroxyphenyl)- benz[e][l,3]oxazin-4-one obtained as per example-3 and methanol (1 L) and heated to 60-65C then stirred for 5 hours at reflux temperature. The reaction mixture was cooled to 0-5 C stirred for 1.5 hours. The solid formed was filtered, washed with methanol and then dried to get the title compound.Yield: 75 grams; Purity by HPLC: 99.77 %; impurity at 2.1 RRT : 0.01%.Particle size distribution: D(0.1):2.40 mum; D(0.5):6.28 mum; D(0.9): 12.98 mum; D(1.00): 21.27 mum. |
|
In ethanol; for 2h;Reflux; |
Example 3; Preparation of 4-[3,5-Bis(2-hydroxyphenyl)-1H-1,2,4-triazol-1-yl]benzoic acid (Deferasirox)2-(2-Hydroxyphenyl)-4H-1,3-benzoxazine-4-one (25 gm, 0.1045 moles) and 4-hydrazinobenzoic acid (17.5 gm, 0.115 moles) were taken in ethanol (375 ml). The reaction mass was heated to reflux and refluxed for 2 hours. The resulting mass was cooled to 25 C., the resulting solid was filtered and dried to produce 30.4 gm of 4-[3,5-Bis(2-hydroxyphenyl)-1H-1,2,4-triazol-1-yl]benzoic acid (Melting point: 264 C.). |
|
In methanol; for 2h;Reflux; |
REFERENCE EXAMPLEPreparation of Deferasirox (Crude)2-(2-Hydroxyphenyl)-4H-1,3-benzoxazin-4-one (25 g, 0.1045 mol), 4-hydrazinobenzoic acid (17.5 g, 0.115 mol) and methanol (375 ml) were added to a round bottom flask. The reaction mixture was heated to reflux temperature and refluxed for 2 hours. The resulting mass was cooled to 25 C. and then filtered to isolate the solid product. The wet material was added to methanol (1625 ml) and heated to 65 to 66 C. to provide a clear solution. The resulting solution mass was cooled to 25 C., and the separated solid was filtered and then dried to produce 28.5 g of crude deferasirox (HPLC Purity: 99.8%, content of 4-hydrazinobenzoic acid impurity: 60 ppm). |
|
With potassium hydrogensulfate; In ethanol; water; at 25 - 75℃; for 2.75h;Industry scale; |
Example- II [73] Preparation of Deferasirox [74] [75] 2-(2-hydroxyphenyl) benz [e] [1, 3] oxazin-4-one (1.0 kg) and 4-Hydrazino benzoic acid (0.699 kg), Ethanol (9.0 lit), Water (1.0 lit) was stirred at 25-350C in round bottom flask. Followed by addition of Potassium hydrogen sulphate (0.15 kg) into the reaction mass and heated the reaction mass at 70-75 0 C for two hrs. Cooled the reaction mass at 25-30 0C and stirred for 45 minutes. Filtered off the solid and washed with ethanol. Obtained solid and water (1.0 lit) stirred at 25-30 C for 15 minutes and then heated at 50- 55 0C. for 1 hr. The solid was filtered at 50-55 0C and washed with water. Charge this wet cake in DMF (5.0 lit) and stirred at 25-30 0C. In this solution 30% H2O2 was added at 25-30 0C within 1 hr. Followed by addition of water at 25-30 0C and stirred for 30 minutes. Filtered off the solid and washed it with water. The obtained solid was added in pre heated water and stirred at 45-50 0C. Gradually increased the temperature up to 65-75 0C & stir it for 60 min. cooled the reaction mass at 45-50 0C and stirred it for 40 minutes. The precipitated Deferasirox filtered off and washed with water. Dry the solid at 65-75 0C in vacuum tray drier. |
|
|
Example 2 Preparation of Deferasirox A mixture of methanol (450.0 ml), 2-(2-hydroxyphenyl)-benz[1,3]oxazin-4-one (30.0 gm) were stir for 10 min at 25-30 C. To the above contents 4-hydrazino benzoic acid (20.03 gm) was added. The contents were heated to reflux temperature 65-70 C. The contents were maintained for 4 hours at 65-70 C. The reaction mass was cooled Slowly to 0-5 C. and maintained it for 1 hour at 0-5 C. The reaction mass was filtered and washed with methanol (30.0 ml). Compound was taken into methylene chloride and stir for 10 min 25-30 C. The contents were heated to reflux temperature (40-45 C.) and maintained the contents for 1 hr at reflux temperature. Cool the contents to 25-30 C. and stirred for 1 hr at 25-30 C. The reaction mass was filtered and washed with methylene chloride (30.0 ml). Dried the compound at 60-65 C. Yield: 79.0%. |
|
With potassium hydrogensulfate; In ethanol; water; at 70 - 75℃; for 2h; |
EXAMPLE-II Preparation of Deferasirox 2-(2-hydroxyphenyl) benz [e] [1, 3] oxazin-4-one (1.0 kg) and 4-Hydrazino benzoic acid (0.699 kg), Ethanol (9.0 lit), Water (1.0 lit) was stirred at 25-35 C. in round bottom flask. Followed by addition of Potassium hydrogen sulphate (0.15 kg) into the reaction mass and heated the reaction mass at 70-75 C. for two hrs. Cooled the reaction mass at 25-30 C. and stirred for 45 minutes. Filtered off the solid and washed with ethanol. Obtained solid and water (1.0 lit) stirred at 25-30C for 15 minutes and then heated at 50- 55 C. for 1 hr. The solid was filtered at 50-55 C. and washed with water. Charge this wet cake in DMF (5.0 lit) and stirred at 25-30 C. In this solution 30% H2O2 was added at 25-30 C. within 1 hr. Followed by addition of water at 25-30 C. and stirred for 30 minutes. Filtered off the solid and washed it with water. The obtained solid was added in pre heated water and stirred at 45-50 C. Gradually increased the temperature up to 65-75 C. & stir it for 60 min. cooled the reaction mass at 45-50 C. and stirred it for 40 minutes. The precipitated Deferasirox filtered off and washed with water. Dry the solid at 65-75 C. in vacuum tray drier. |
|
With triethylamine; In ethanol; at 82℃; |
1.75 g of 4-hydrazinobenzoic acid(e) and 1.16 g of NEt3 were completely dissolved in 30 mL of hot ethanol solution. Add 2.50g of key intermediate a, The reaction was heated to reflux for 2-3 h. After the reaction is over, Concentrate to half volume under reduced pressure, Add the right amount of water, Adjust the pH to 5 with hydrochloric acid, There are white flocs, Filtered to purify a pale yellow solid. Melting point 261-262 C. |
| 13 g |
In ethanol; for 2h;Reflux; |
10 grams of 2-(2-Hydroxyphenyl)-4H- l ,3-benzoxazin-4-one (Deferasirox cyclic compound) and 6.5 grams of 4-hydrazinobenzoic acid were boiled under reflux temperature for 2 hours in 70 ml absolute alcohol. The product was precipitated during reflux and cooled to room temperature, filtered and washed with absolute alcohol (2x10 ml). The obtained wet Deferasirox (16grams) was dissolved in absolute alcohol (430 ml) at reflux temperature, charcolized, filtered through hyflo bed at hot condition and then washed with hot absolute alcohol (20 ml). Filtrate was distilled off until the mass volume reaches about 100 ml and cooled to room temperature. The precipitated product was filtered and washed with absolute alcohol. The product was dried at 50-55 C for 6 hours, obtained about 13 grams of pure 4-[3,5-Bis (2-hydroxyphenyl)- lH- l ,2,4-triazol-l-yl]benzoic acid [HPLC Purity: 99.95 %; 'hydrazino impurity' : l lO ppm]. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping