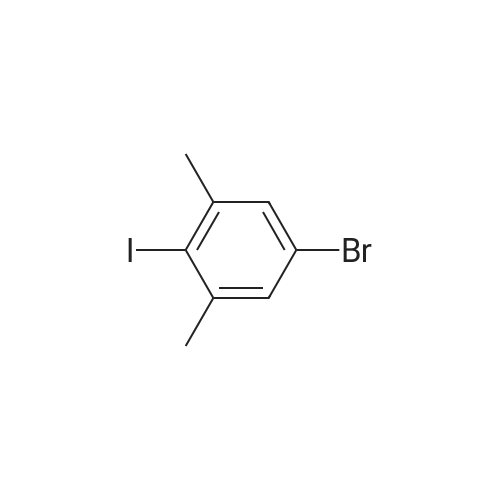
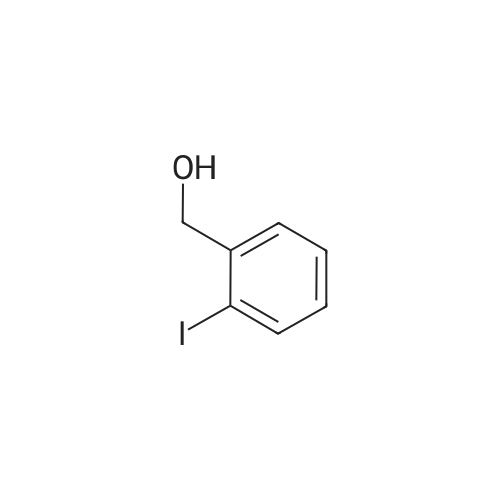
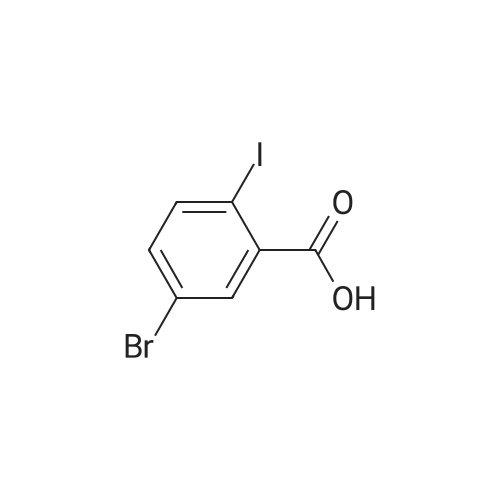
|
With oxalyl dichloride; dimethyl sulfoxide; triethylamine; In dichloromethane; at -70 - -10℃; for 1.25h; |
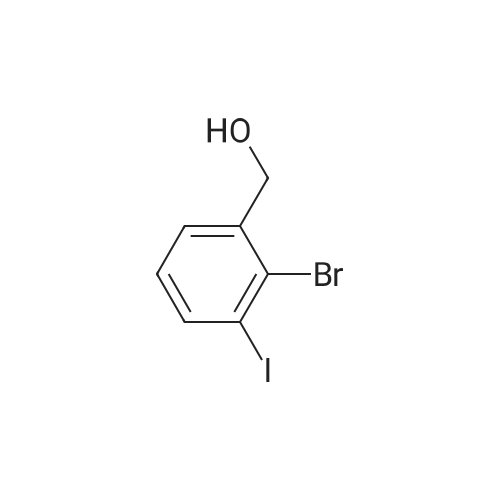
A solution of oxalyl chloride (1.53 g, 0.012 mol) in CH2Cl2 (15 mL) was cooled to -70 C., and DMSO (1.41 g, 0.018 mol) in CH2Cl2 (15 mL) was added at -65 to -70 C. The mixture was stirred under nitrogen for 10 minutes at -70 C. and then treated with the product from Example 15B (2.35 g, 7.5 mmol) in 60 mL CH2Cl2. The slurry was stirred at -65 C. for 15 minutes and treated with triethylamine (3.8 g, 0.037 mol). The mixture was allowed to warm to -10 C. over 1 hour. The mixture was treated with 20 mL of water and allowed to warm to room temperature. The organic layer was separated and concentrated to provide the title compound. 1H NMR (CDCl3, 400 MHz) δ 9.97 (s, 1H), 7.97 (d, J=4 Hz, 1H), 7.79 (d, J=8 Hz, 1H), 7.40 (dd, J=4, 8 Hz, 1H). MS (DCl/NH3) [M+NH4]+ at 328. |
|
|
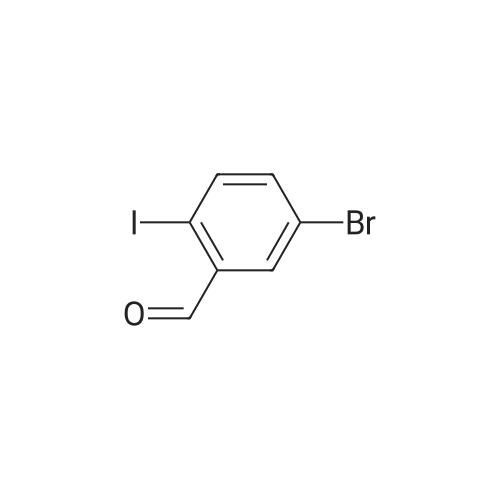
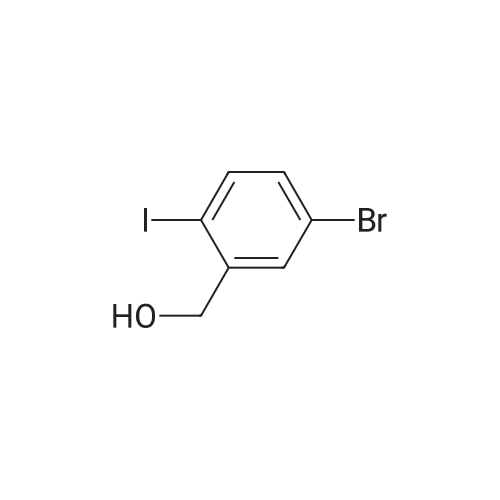
Example 15C 5-Bromo-2-iodobenzaldehyde A solution of oxalyl chloride (1.53 g, 0.012 mol) in CH2Cl2 (15 mL) was cooled to -70 C., and DMSO (1.41 g, 0.018 mol) in CH2Cl2 (15 mL) was added at -65 to -70 C. The mixture was stirred under nitrogen for 10 minutes at -70 C. and then treated with the product from Example 15B (2.35 g, 7.5 mmol) in 60 mL CH2Cl2. The slurry was stirred at -65 C. for 15 minutes and treated with triethylamine (3.8 g, 0.037 mol). The mixture was allowed to warm to -10 C. over 1 hour. The mixture was treated with 20 mL of water and allowed to warm to room temperature. The organic layer was separated and concentrated to provide the title compound. 1H NMR (CDCl3, 400 MHz) δ 9.97. (s, 1H), 7.97 (d, J=4 Hz, 1H), 7.79 (d, J=8 Hz, 1H), 7.40 (dd, J=4, 8 Hz, 1H). MS (DCl/NH3) [M+NH4]+ at 328. |
|
With dipyridinium dichromate; In dichloromethane; at 20℃; for 4h; |
The compound was synthesized according to the published procedure. [Zhou, N.; Wang, L.; Thompson, D. W.; Zhao, Y. Org. Lett. 2008, 10 (14), 3001-3004] Compound L15 and pyridinium dichromate (7.2 g, 19.2 mmol, 2 eq.) were dissolved in dry dichloromethane (40 ml) and the mixture was stirred at room temperature for 4 hours. The mixture was filtered through celite, washed with diethyl ether and solvents were evaporated. The solid was adsorbed on silica gel in a mixture of cyclohexane/acetone and it was purified by flash column chromatography on silica gel (cyclohexane to 20% ethyl acetate modified with 10% methanol (v/vi)), yielded 2.3 g (77%) of the title compound as a white solid (85% NMR purity). 1H NMR (401 MHz, Chloroform-d) δ 9.99 (s, 1H), 7.99 (d, J= 2.5 Hz, 1H), 7.81 (d, J= 8.4 Hz, 1H), 7.41 (dd, J= 8.4, 2.5 Hz, 1H). 13C NMR (101 MHz, Chloroform-d) δ 194.45, 141.95, 138.37, 136.47, 133.24, 123.63, 98.39. MS (CI-QMS) m/z: [M + H]+ calcd for C7H5BrIO, 310.9; found, 310.9. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping