| 100% |
|
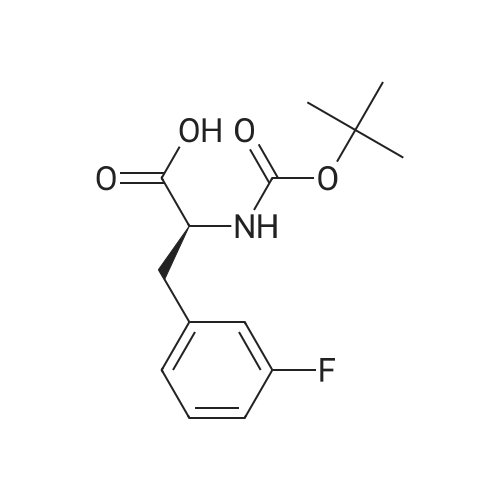
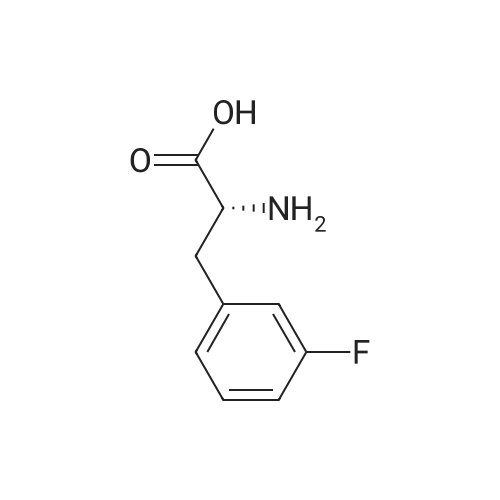
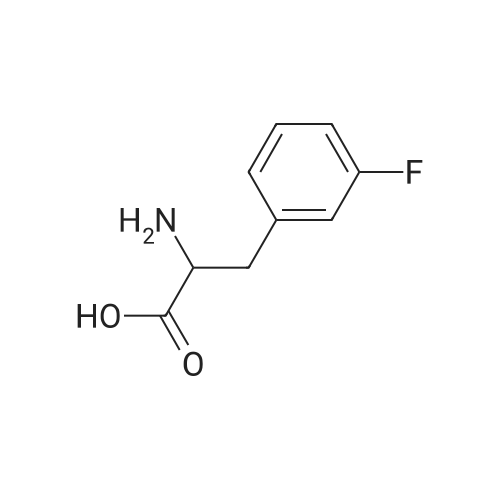
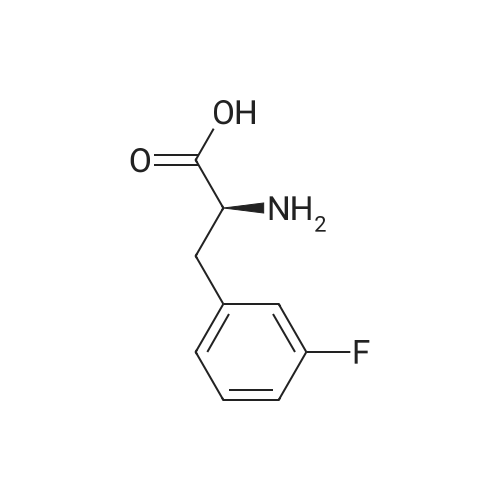
A mixture of L-2-AMINO-3- (3-FLUORO- phenyl)-propionic acid (20.0 g, 110 MMOL, 1 eq) in H20 (100 mL) was treated with NA2CO3 (16.2 g, 153 mmol, 1.4 eq) in H20 (40 mL) followed by 1,4-dioxane (100 mL) and cooled to 0 C. The BOZO was added and the reaction mixture was stirred at ambient temperature for 5 h after which the- dioxane was evaporated. H2O (125 mL) was then added and the mixture then washed with Et2O (2 x 100 mL). The aqueous phase was acidified with 10% citric acid followed by extraction with EtOAc (2 x 300 mL). The combined EtOAc layers were washed with H20 (2 x 150 mL), brine (150 mL), dried (Na2SO4) and concentrated to give the acid as a colorless, viscous oil which slowly solidified upon standing (31 G, QUANT). H NMR (CDC13) 7.33-7. 26 (m, 1H), 7.00-6. 91 (m, 3H), 4.96 (s, 1H), 4.62 (bs, 1H), 3.23 (dd, J=14, 5.3, 2H), 1. 44 (s, 9H) ; Anal CALCD for C14HL8NO4F : C, 59.36 ; H, 6.40 ; N, 4.94. Found: C, 59.29 ; H, 6.34 ; N, 4.90. |
| 100% |
With sodium carbonate; In 1,4-dioxane; water; at 0 - 25℃; for 5h; |
A mixture of BOC-L-3-fluorophenylalanine 1 (20 g, 109 MMOL) in water was treated with sodium carbonate (16.2 g, 15.3 MMOL) in H20 (40 mL). 1,4-Dioxane (100 mL) was added, and the mixture cooled to 0 C. The BOC20 (28. 6 g, 120 MMOL) was added in one portion, and the mixture was maintained for 5 h at 25 C. The solvent was evaporated and H2O (125 mL) was added. The aqueous layer was washed with diethyl ether (2 x 100 mL). The ether layers were discarded, and the aqueous layer was acidified with a 10% citric acid solution. The mixture was then extracted with EtOAc (2 x 150 mL). The organic layers were combined, washed with H20 (2 x 150 mL), Brine (150 mL), dried (NA2SO4), filtered and evaporated to give the desire crude product 2 as a clear viscous oil. 30.9 9, 100%, ) which slowly solidified to a white solid at rt. 1H NMR (300 MHz, CDCl3) 8 7.33-7. 26 (M, 1H), 7.00-6. 91 (m, 3H), 4.96 (s, 1H), 4.62 (bs, 1H), 3.23 (dd, J= 14,5. 3 Hz, 2H), 1.44 (s, 9H) ppm; Anal CALCD for CR4HR8NO4F : C, 59.36 ; H, 6.40 ; N, 4.94. Found: C, 59.29 ; H, 6.34 ; N, 4.90. |
| 97% |
With sodium hydrogencarbonate; In tetrahydrofuran; water;pH 9.0; |
3-Fluoro-L-Phenylalanine (1 g, 5.46 mmol, Combi-blocks, Cat SS-0819, Lot L78093) was dissolved in THF (15 mL) and water (15 mL) and the pH of the solution was adjusted to 9 using saturated sodium bicarbonate solution. Di -tert- butyl -di carbonate (1.31 g, 6 mmol) was added to the solution slowly and was stirred overnight. After completion of the reaction, the pH of the solution was adjusted to 4 using 0.1 M HC1 and the aqueous layer was extracted with ethyl acetate. Combined organic layers were washed with water (x2) followed by brine, dried using sodium sulfate, filtered and concentrated under reduced pressure to afford 1.5 g (97% yield) off-white solid of the title product (Int-1). LCMS (+ESI) M+H+(-Boc)=l 84.1. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping