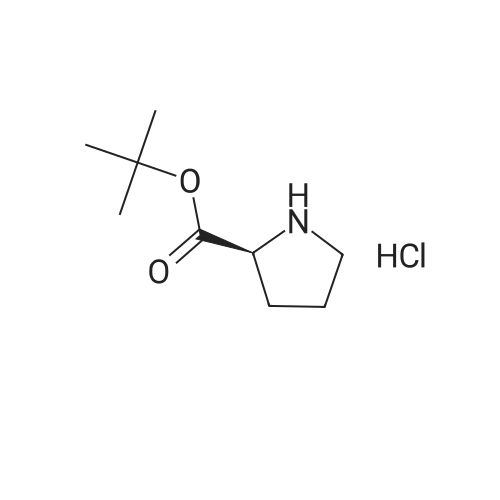
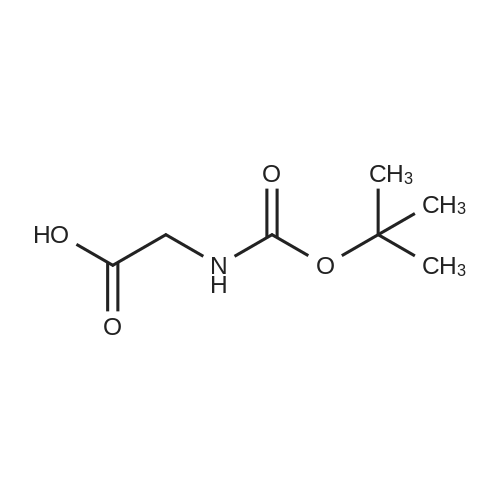
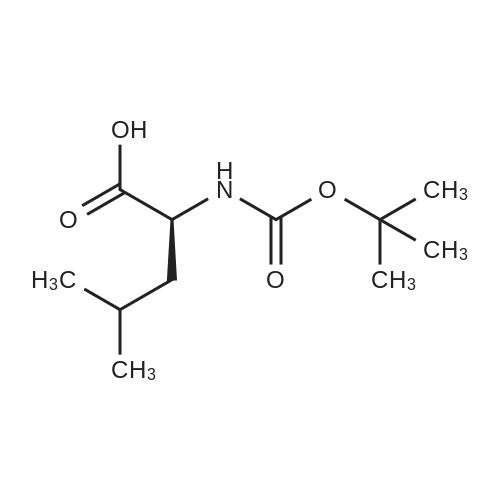
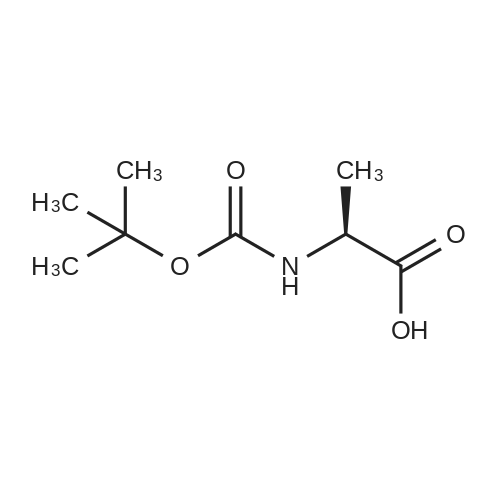
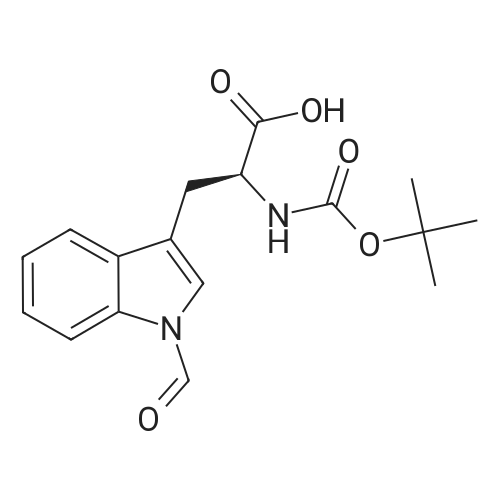
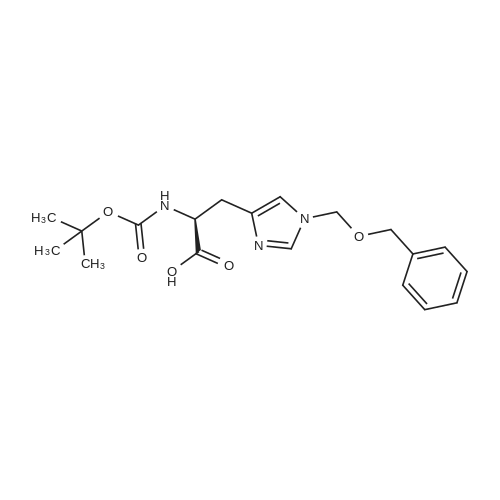
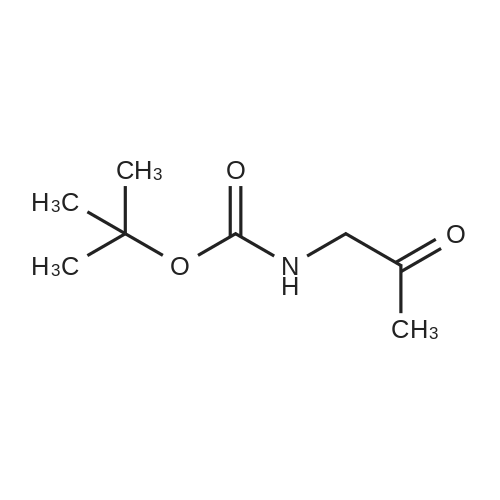
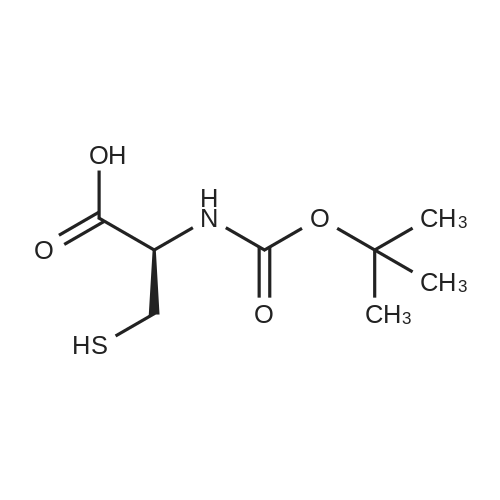
|
|
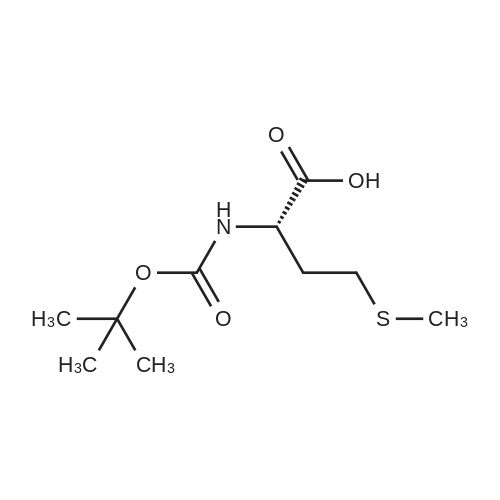
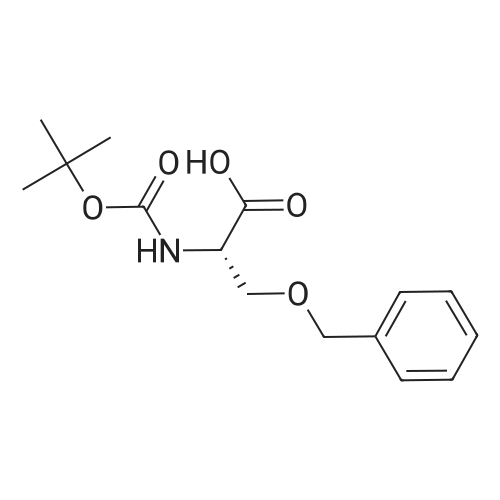
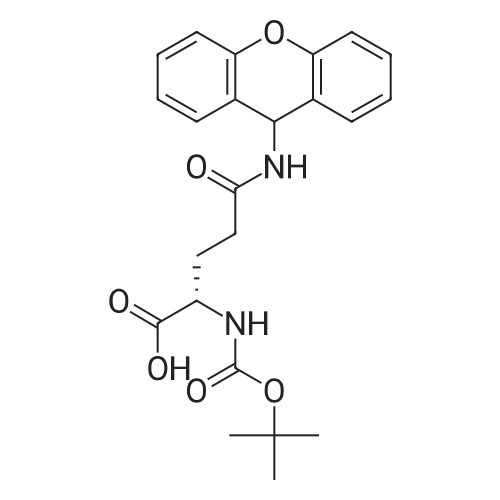
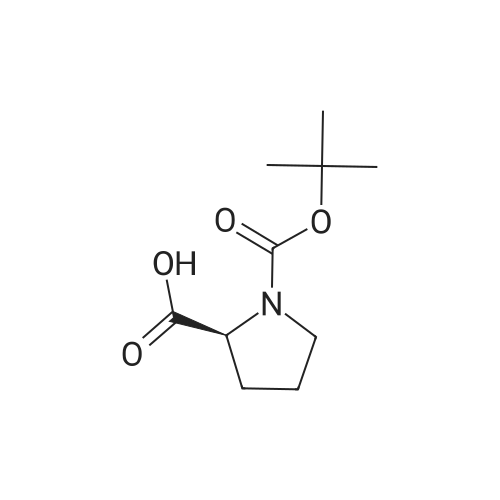
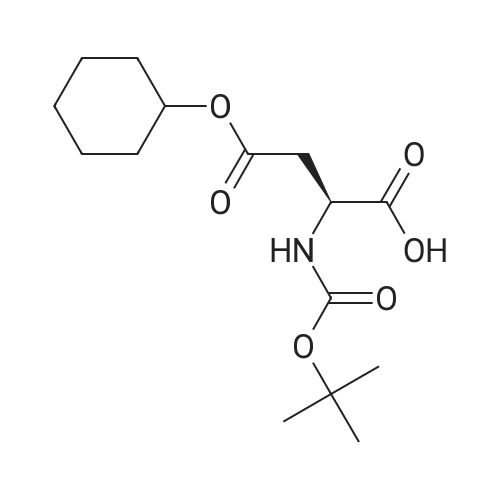
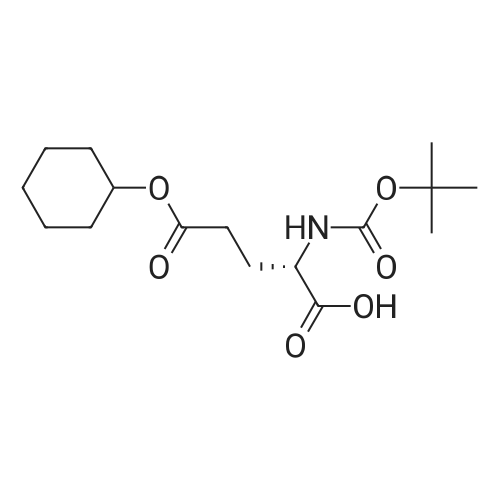
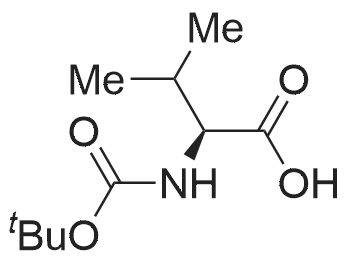
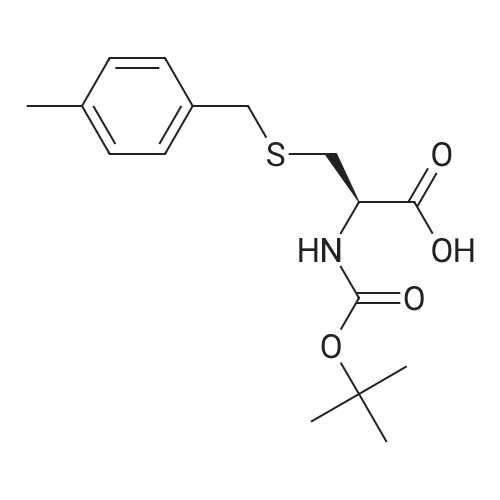
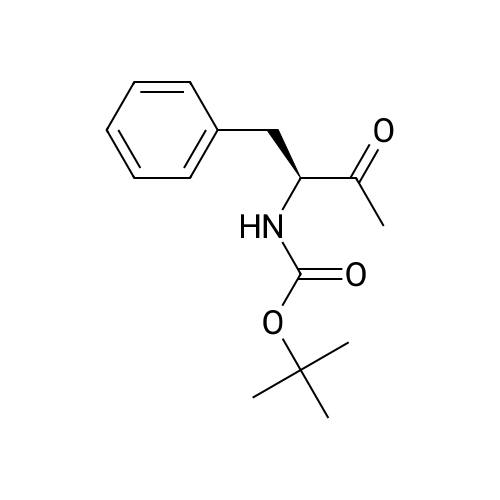
1.43 g of Boc-Ile-PAM resin (0.70 mmol/g, 1 mmol) was swollenin DMF for 1 hour, after which the terminal Boc group was removedwith 2 1 min treatment with neat TFA. The resin wasflow-washed with DMF for 30 s, and neutralized with 2 1 mintreatments with 10% DIEA in DMF (v/v). The neutralized resinwas then flow washed for 30 s with DMF, then 30 s with DCM.10 equiv of DCC (2.063 g, 10 mmol) were dissolved in 5 mL ofDCM and 20 equiv of 3-iodopropionic acid (4.0 g, 20 mmol) weredissolved in 6 mL of DCM. Both solutions were cooled to 0 Cfor 5 min before being combined and allowed to react at 0 C for30 min. The resulting solution containing 3-iodopropionic anhydridewas gravity filtered to remove the dicyclohexylurea precipitate.The residue was then washed with 2 mL of DCM, filtered, andpooled with the 3-iodopropionic anhydride filtrate. The pooled filtrateswere then added to the deprotected neutralized Ile-PAM resinand allowed to couple for 45 min. The resin was drained andwashed with DCM for 30 s. Quantitative ninhydrin test indicated>99% coupling. The resin was then dried under vacuum. Yield:1.49 g (new loading: 0.661 mmol/g, 0.984 mmol) yellowish resin.550 mg (0.661 mmol/g, 0.363 mmol) of the dried acylated resinwas then swollen in 10 mL of anhydrous THF for 1 h. 5 equiv ofKSeCN (262 mg, 1.82 mmol) were added to the swollen resin inTHF. The mixture was then incubated under argon atmosphere at45 C and gently agitated for 22 h (magnetic stirring should be avoided as the stir bar will crush the resin which will later clog theSPPS synthesis vessel frits). The resin was drained and washed extensively with THF and DCM and dried under vacuum. Yield:452 mg (new loading: 0.67 mmol/g, 0.303 mmol) of greyish-whiteresin.The dried resin (450 mg, 0.3 mmol) was re-swollen in 8 mL ofTHF for 1 h, cooled to 0 C on an ice/water bath, and sodium borohydride(37.83 mg, 1 mmol) in 95% (v/v) EtOH/H2O (1 mL) wasadded in one portion. The reaction was placed at 0 C for 1 h withoccasional agitation, drained, and washed with THF, DCM, andDMF.Prior to coupling of the first amino acid, the resin was treatedwith 3 mmol 1,4-dithiothreitol (DTT) in 6 mL DMF for 10 min.The resin was then drained, and without washing, a 10 equivpre-activated mixture of Boc-Xaa-OH (3 mmol), HATU (1140 mg,3 mmol), and 1.038 mL DIEA (6 mmol) in 6 mL of DMF was addedand allowed to couple for 1 h. The resin was then drained andwashed with DMF, and the reduction and coupling steps were performeda second time. The resin was washed with DMF and stepwisesynthesis was subsequently carried out using standard Boc in-situ neutralization protocols as described previously. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping