|
With hydrogenchloride;palladium; In tetrahydrofuran; diethyl ether; |
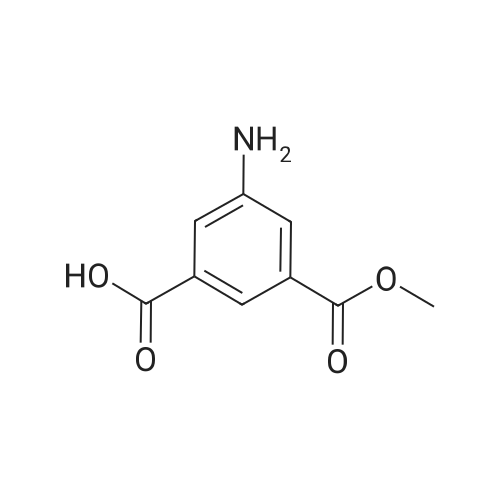
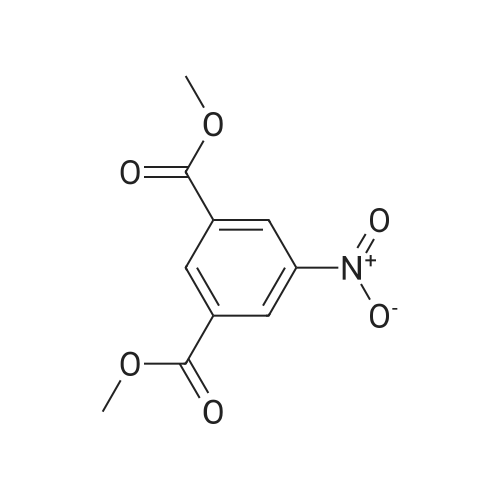
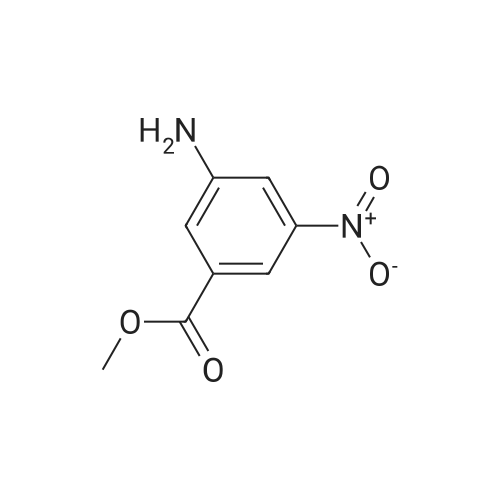
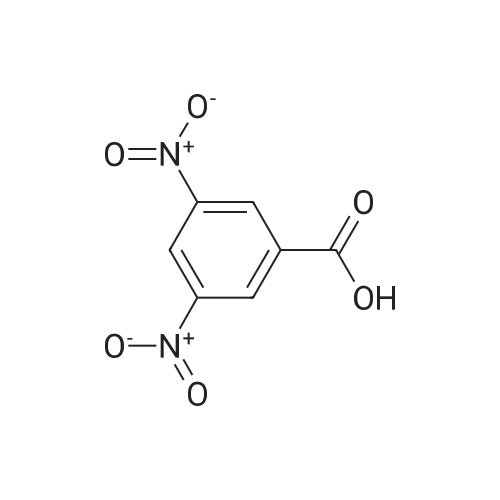
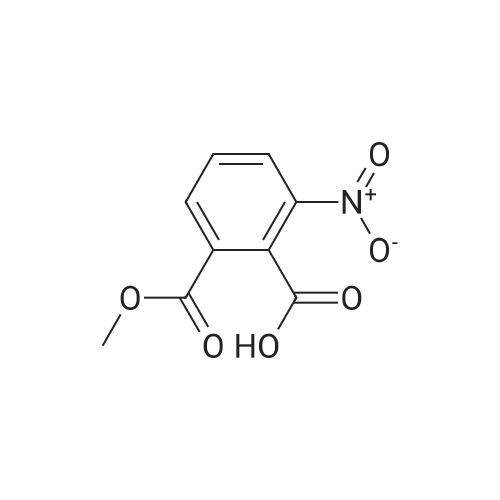
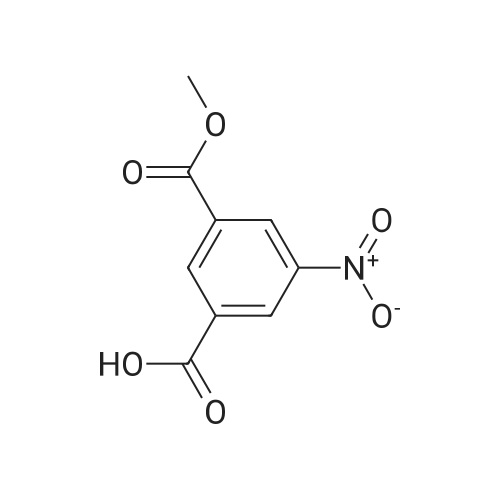
Preparation of 3-Amino-5-(methoxycarbonyl)benzoic Acid (Compound 68) To 2.75 g of mono-methyl 5-nitroisophthalate (5-(methoxycarbonyl)-3-nitrobenzoic acid), compound 67, dissolved in 30 mL of THF was added 100 mg of 10% palladium on carbon. The reaction was placed in a Parr hydrogenator under a H2 atmosphere of 45 psi and shaken for 16 hr. The solid palladium catalyst was removed by vacuum filtration through celite and 5 mL of 1N HCl in diethyl ether was added to the filtrate. After sitting for 12 hr, the solid was collected by vacuum filtration and was washed with ethyl acetate. This provided 1.82 g of the desired product. The product was identified by 1H NMR and mass spectroscopy and purity was assessed by RP-HPLC. |
|
With hydrogen;palladium on activated charcoal; In tetrahydrofuran; methanol; under 750.075 Torr; |
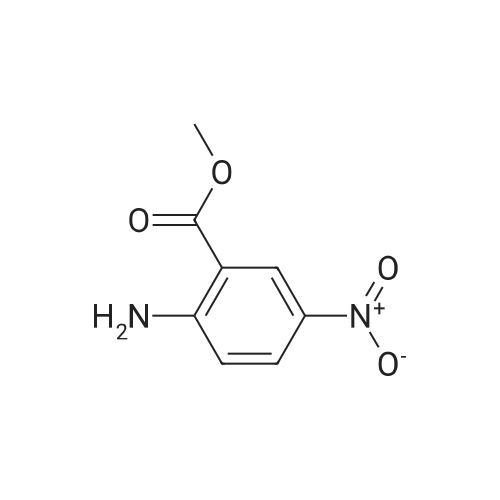
Monomethyl-5-nitroisophthalate (50 g, 220 mmol, 1 eq) is dissolved in a mixture of 650 ml of MeOH and 350 ml of THF. 3 g of Pd/C are added, and the reaction mixture is hydrogenated overnight under 1 bar of H2 and then filtered. The filtrate is concentrated, and the residue is dissolved in a mixture of THF (200 ml) and aq NaHCO3 (400 ml). CbzCI (62 ml, 50% in toluene, 184 mmol, 0.9 eq) is added, the reaction mixture is stirred for 1 h, CbzCI (31 ml, 50% in toluene, 92 mmol, 0.45 eq) is added, and the reaction mixture is stirred overnight. The white solid formed is washed with water and diethyl ether to give the product.MS (ES-): 328 = [M-HT.HPLC (Nucleosil C18HD, 4x70 mm, 3 mum, 20-100% MeCN (6 min), 100% MeCN (1.5 min)) retention time: 4.36 min.1H-NMR (400 MHz, DMSO-d6): 8.40 (s, 1H), 8.38 (s, 1H), 8.17 (s, 1H), 7.50-7.37 (m, 5H),5.21 (s, 2H), 3.92 (s, 3H). |
|
With hydrogen;palladium on activated charcoal; In tetrahydrofuran; methanol; under 750.075 Torr; |
a) 5-Benzyloxycarbonylamino-isophtalic acid monomethylesterMonomethyl-5-nitroisophtalate (50 g, 220 mmol, 1 eq) is dissolved in a mixture of 650 ml of MeOH and 350 ml of THF. 3 g of Pd/C are added, and the reaction is hydrogenated over night under 1 bar of hydrogen. The reaction mixture is then filtered and concentrated to give the amine as a crude product, which is then dissolved in a mixture of THF (200 ml) and <n="37"/>aqueous sodium bicarbonate (400 ml). CbzCI (62 ml, 50% in toluene, 184 mmol, 0.9 eq) are added to the reaction mixture, and the reaction is stirred for 1 hour. CbzCI (31 ml, 50% in toluene, 92 mmol, 0.45 eq) are added, and the reaction is stirred over night. The white solid which crashes out, is washed with water and diethyl ether to give the product. 1 H-NMR (400 MHz, dmso-d6): 8.40 (s, 1 H), 8.38 (s, 1 H), 8.17 (s, 1 H), 7.50-7.37 (m, 5H), 5.21 (s, 2H), 3.92 (s, 3H). |
|
With hydrogen;palladium on activated carbon; In methanol; under 2585.81 Torr; for 3.0h; |
Step G (I): 3-ammo-5-(methoxycarbonyl)benzoic acid.; A suspension of 3-(methoxycarbonyl)-5-nitrobenzoic acid (20.0 g) and palladium on carbon (5 wt%, 4.0 g) in MeOH (600 mL) was shaken in hydrogenator under hydrogen at 50 psi for 3 h. The mixture was filtered and concentrated in vacuo to give the EPO <DP n="42"/>title compound: 1H NMR (CD3OD, 500 MHz) delta ppm 3.90 (3H, s), 7.52 (IH5 m), 7.55 (IH, m), 7.92 (IH, m). MS (ESI) (M-H)" 194.08. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping