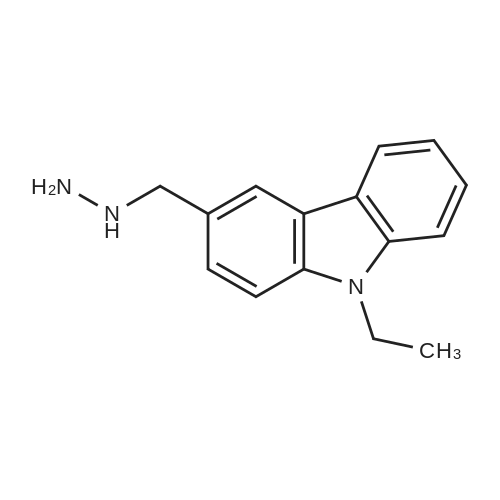
Alternatived Products of [ 19402-87-0 ]
Product Details of [ 19402-87-0 ]
| CAS No. : | 19402-87-0 |
MDL No. : | MFCD00218286 |
| Formula : |
C19H15N
|
Boiling Point : |
No data available |
| Linear Structure Formula : | - |
InChI Key : | HBAKJBGOHINNQM-UHFFFAOYSA-N |
| M.W : |
257.33
|
Pubchem ID : | 146485 |
| Synonyms : |
|
Application In Synthesis of [ 19402-87-0 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 19402-87-0 ]
- 1
-
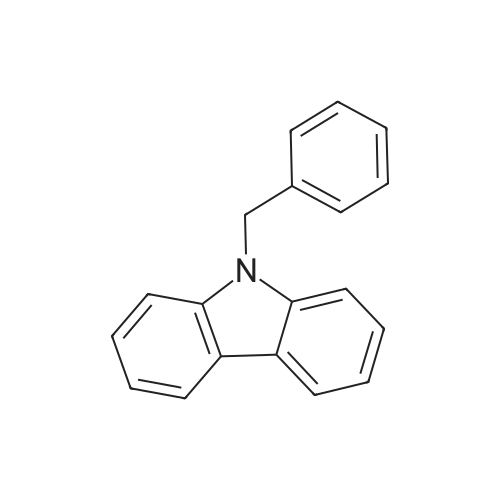 [ 19402-87-0 ]
[ 19402-87-0 ]

-
 [ 68-12-2 ]
[ 68-12-2 ]

-
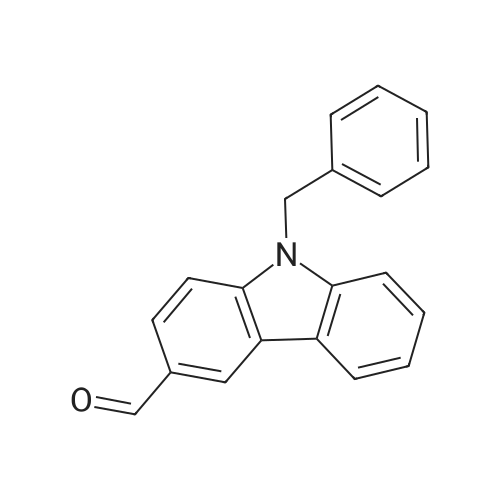 [ 54117-37-2 ]
[ 54117-37-2 ]
| Yield | Reaction Conditions | Operation in experiment |
| 80% |
With trichlorophosphate; In chloroform; at 0 - 90℃; for 8h; |
General procedure: DMF (0.4 ml)was added into a dried round-bottom flask and thesystem was cooled to 0 C. A solution of CHCl3 (3 ml) containing1ae1i (5 mmol) was then added following the addition of redistilled POCl3 (0.45 mL). Then, the solution mixture was heated to 90 C for 8 h. After most of the CHCl3 was removed the residue waspoured into ice water and the pH was then adjusted to 7-8 using NaHCO3, the water layer was extracted with CH2Cl2 and organiclayer was washed with water several times before being dried with Mg2SO4, after CH2Cl2 was removed, the crude product was purifiedby column chromatography using ethyl acetate/petroleum ether(1:10, V/V) as an eluent, and finally a primrose solid was obtainedfor 2a-2i in 62e81% yields. |
| 80% |
With trichlorophosphate; at 0 - 90℃; for 8h; |
General procedure: DMF (0.4 ml) was added into a dried round-bottom flask and the system was cooled to 0 C. A solution of CHCl3 (3 ml) containing 1a-1m(5 mmol) was then added following the addition of redistilled POCl3 (0.45 mL).Then, the solution mixture was heated to 90 C for 8 h. After most of the CHCl3 was removed the residue was poured into ice water and the pH was then adjusted to 7-8 using NaHCO3, the water layer was extracted with CH2Cl2 and organic layer was washed with water several times before being dried with Mg2SO4, after CH2Cl2 was removed, the crude product was purified by column chromatography using ethyl acetate/petroleum ether (1:10, V/V) as an eluent, and finally a primrose solid was obtained for 2a-2m in 62-81% yields. |
| 36.1% |
|
Step 2. 9-Benzyl-9H-carbazole-3-carbaldehyde; A 50-mL 3-necked round-bottomed flask was charged with N,N-dimethylformamide (400 mg, 5.42 mmol, 2.80 equiv, 99%). To this, POCl3 (700 mg, 4.56 mmol, 2.40 equiv, 99%) was added drop wise with stirring at 0 C. and allowed to stir at room temperature for 1 hour. To this mixture was added 9-benzyl-9H-carbazole (500 mg, 1.93 mmol, 1.00 equiv, 99%) in small portions at 45 C. over 5 minutes. Then, the temperature was raised to 95 C. in an oil bath and allowed to stir for 18 hours. The progress was monitored by TLC (EtOAc:PE=1:4). Upon completion, the reaction mixture was cooled down to room temperature and quenched with water (20 mL). The resulting mixture was allowed to stir for an additional 4 hours at room temperature. The solids were collected by filtration and dried to afford 9-benzyl-9H-carbazole-3-carbaldehyde as green solid (200 mg, 36.1%). LCMS: [M+H]+: 286 |
| 2.64 g |
With trichlorophosphate; at 80℃; for 3h;Cooling with ice; |
In a 100mL three-necked flask equipped with a magnetic stirrer, 3g (12mmol) of the prepared N-benzylcarbazole dissolved in 10mL DMF was added. The mixture was subjected to an ice bath. 4mL POCl3 was slowly added dropwise. After addition of POCl3, the solution was cooled at room temperature giving a pale yellow solution. The solution was heated at 50C for 20min then temperature was raised to 80C. The solution turned dark red. the reaction was continued 3h forming a precipitate. The reaction was stopped. The mixture was filtered and dried to give 2.64g of N-benzylcarbazol-3-aldehyde as a pale yellow solid. |
| 5.41 g |
With trichlorophosphate; at 0 - 20℃; for 17h;Reflux; |
A solution of CHCl3 (25 mL) containing 9-benzyl-9H-carbazole (6.43 g, 25 mmol) and DMF(1.86 mL) was cooled to 0C. POC13 (2.3 mL) was slowly added at 0C and the solution was allowedto stir to room temperature for one hour. Then, the solution mixture was refluxed for 16 h. Duringreaction, a yellow precipitate formed. After cooling, the solution was poured into ice water. Theyellow solid was filtered off, washed with ether and pentane, and dried under vacuum. The crudeproduct was recrystallized in ethanol, and cooled at -30C to end the precipitation (5.41 g, 76%yield). |

Reference:
[1]European Journal of Medicinal Chemistry,2018,vol. 145,p. 498 - 510
[2]Bioorganic and Medicinal Chemistry Letters,2018,vol. 28,p. 1320 - 1323
[3]Journal of Polymer Science, Part A: Polymer Chemistry,2017,vol. 55,p. 1189 - 1199
[4]Patent: US8080566,2011,B1 .Location in patent: Page/Page column 41
[5]Canadian Journal of Chemistry,1990,vol. 68,p. 908 - 925
[6]Patent: CN105348277,2016,A .Location in patent: Paragraph 0012
[7]Molecules,2017,vol. 22
- 2
-
 [ 19402-87-0 ]
[ 19402-87-0 ]

-
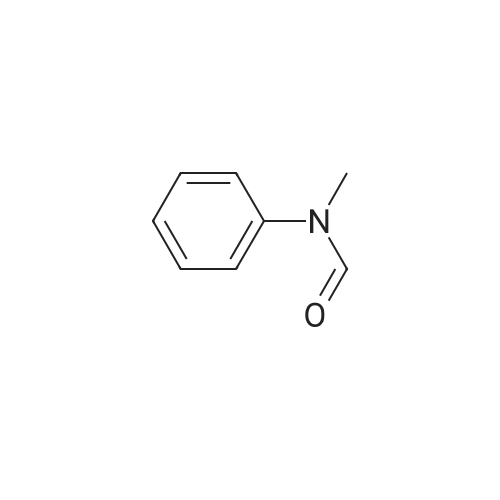 [ 93-61-8 ]
[ 93-61-8 ]

-
 [ 54117-37-2 ]
[ 54117-37-2 ]
- 3
-
 [ 19402-87-0 ]
[ 19402-87-0 ]

-
 [ 4885-02-3 ]
[ 4885-02-3 ]

-
 [ 54117-37-2 ]
[ 54117-37-2 ]

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping