Alternatived Products of [ 18995-35-2 ]
Product Details of [ 18995-35-2 ]
| CAS No. : | 18995-35-2 |
MDL No. : | MFCD04038411 |
| Formula : |
C10H13ClO
|
Boiling Point : |
No data available |
| Linear Structure Formula : | - |
InChI Key : | NEJWTQIEQDHWTR-UHFFFAOYSA-N |
| M.W : |
184.66
|
Pubchem ID : | 140458 |
| Synonyms : |
|
Safety of [ 18995-35-2 ]
| Signal Word: | Warning |
Class: | |
| Precautionary Statements: | P280-P305+P351+P338 |
UN#: | |
| Hazard Statements: | H302 |
Packing Group: | |
| GHS Pictogram: |

|
Application In Synthesis of [ 18995-35-2 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 18995-35-2 ]
- 1
-
 [ 507-20-0 ]
[ 507-20-0 ]

-
 [ 106-48-9 ]
[ 106-48-9 ]

-
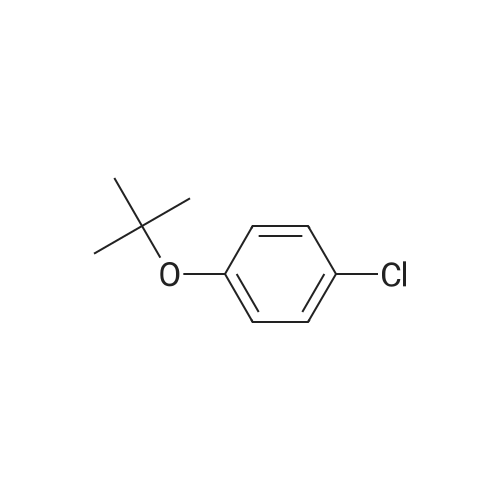 [ 18995-35-2 ]
[ 18995-35-2 ]
- 2
-
 [ 6669-13-2 ]
[ 6669-13-2 ]

-
 [ 18995-35-2 ]
[ 18995-35-2 ]
- 5
-
 [ 27607-77-8 ]
[ 27607-77-8 ]

-
 [ 18995-35-2 ]
[ 18995-35-2 ]

-
tris(4-tert-butoxyphenyl)sulfonium trifluoromethanesulfonate
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 45% |
With thionyl chloride; ammonium chloride; magnesium; In tetrahydrofuran; chloroform; water; |
Example 1 Synthesis of tris(4-tert-butoxyphenyl)sulfonium trifluoromethanesulfonate A Grignard reagent was conventionally prepared using 60.7 g (2.5 mol) of magnesium, 461 g (2.5 mol) of <strong>[18995-35-2]4-tert-butoxyphenyl chloride</strong>, and 700 g of THF. The Grignard reagent solution was cooled in an ice water bath whereupon 59.5 g (0.5 mol) of thionyl chloride diluted with 100 g of THF was added dropwise at less 30 C. Reaction mixture was ripened for about 30 minutes. Thereafter, 277.8 g (1.25 mol) of trimethylsilyltrifluoromethane sulfonate was added dropwise at less 20 C. Reaction mixture was further ripened for 1 hour and thereafter, the reaction solution was allowed to stand overnight at room temperature. After the reaction solution was again cooled in an ice water bath, 1,800 g of 16.7% ammonium chloride aqueous solution (NH4 Cl 300 g+H2 O 1,500 g) was added thereto at a temperature not in excess of 30 C. After separation, 1,000 g of chloroform was added to the organic layer, which was washed three times using 1,000 g of water. Thereafter, the solvent was distilled off under reduced pressure by means of a rotary evaporator and the resulting oily residue was recrystallized, isolating tris(4-tert-butoxyphenyl)sulfonium trifluoromethanesulfonate of 99% purity in an amount of 141 g (yield 45%). |
- 6
-
 [ 18995-35-2 ]
[ 18995-35-2 ]

-
 [ 170632-59-4 ]
[ 170632-59-4 ]
| Yield | Reaction Conditions | Operation in experiment |
| 60% |
With thionyl chloride; In tetrahydrofuran; dichloromethane; water; |
Synthesis Example 1 Synthesis of bis(p-tert-butoxyphenyl)sulfoxide A Grignard reagent was prepared in a conventional manner using 24.3 g (1 mol) of metallic magnesium, 203.2 g (1.1 mol) of p-tert-butoxyphenyl chloride and 280 g of THF. The Grignard reagent was diluted with 500 g of THF and cooled below -60 C. with a dry ice methanol bath. To the Grignard reagent solution, a solution of 47.5 g (0.4 mol) of thionyl chloride diluted with 70 g of THF was added dropwise over one hour at a temperature not exceeding 0 C. Stirring was continued for one hour on the ice water bath and 36 g of water then added to decompose the excess of Grignard reagent. To the reaction solution were added 1000 g of methylene chloride, 400 g of saturated ammonium chloride aqueous solution and 300 g of water. After layer separation, the organic solvent layer was washed twice with 700 g of pure water. The organic solvent layer was dried over magnesium sulfate, filtered, and evaporated in vacuo. The resulting oily product was recrystallized, recovering 83 g (yield 60%) of the end product, bis(p-tert-butoxyphenyl)sulfoxide as a white crystal having a purity of 96% and a melting point of 80-82 C. |
- 7
-
 [ 74-96-4 ]
[ 74-96-4 ]

-
 [ 18995-35-2 ]
[ 18995-35-2 ]

-
p-tert-butoxyphenylmagnesium chloride
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
In tetrahydrofuran; |
Example 1 In a one-liter four-necked flask equipped with a stirrer and a reflux condenser, purged with nitrogen and dried was placed 29.2 g (1.2 mol.) of metallic magnesium flakes to which were added 50 ml of anhydrous tetrahydrofuran and 2 ml of ethyl bromide. After confirming initiation of the reaction by bubbling on the surface of the metallic magnesium, a solution of 184.6 g (1.0 mol.) of p-tert-butoxychlorobenzene in 500 ml of anhydrous tetrahydrofuran was added dropwise at the refluxing temperature over approximately 2 hours followed by stirring for 5 hours to give p-tert-butoxyphenylmagnesium chloride. When an aliquot portion of the reaction mixture was taken, decomposed by pouring water and analyzed by gas chromatography, conversion ratio of the Grignard reagent was found to be 99.8%. |
- 8
-
 [ 102-54-5 ]
[ 102-54-5 ]

-
 [ 18995-35-2 ]
[ 18995-35-2 ]

-
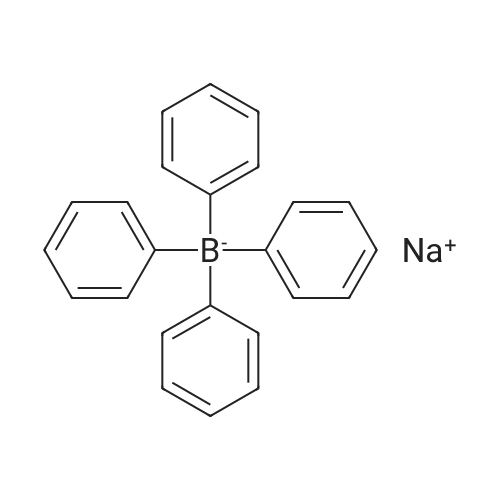 [ 143-66-8 ]
[ 143-66-8 ]

-
(η6-4-chlorophenyl-tert-butyl ether)(η5-cyclopentadienyl)iron(II) tetraphenylborate
[ No CAS ]
- 9
-
 [ 18995-35-2 ]
[ 18995-35-2 ]

-
 [ 590-86-3 ]
[ 590-86-3 ]

-
 [ 1070716-48-1 ]
[ 1070716-48-1 ]
- 10
-
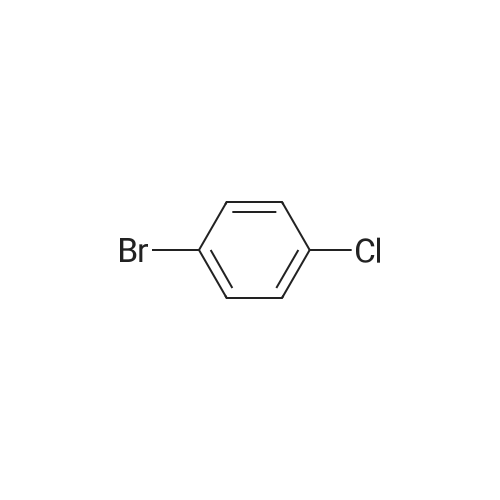 [ 106-39-8 ]
[ 106-39-8 ]

-
 [ 865-47-4 ]
[ 865-47-4 ]

-
 [ 18995-35-2 ]
[ 18995-35-2 ]
- 11
-
(1-(phenylsulfonyl)-1H-indol-2-yl)boronic acid
[ No CAS ]

-
 [ 18995-35-2 ]
[ 18995-35-2 ]

-
 [ 1158985-06-8 ]
[ 1158985-06-8 ]
- 12
-
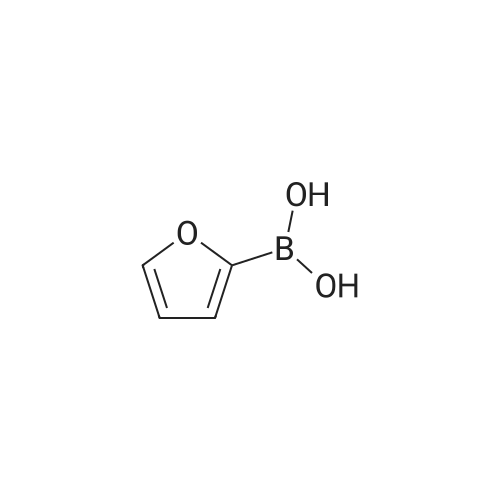 [ 13331-23-2 ]
[ 13331-23-2 ]

-
 [ 18995-35-2 ]
[ 18995-35-2 ]

-
 [ 1158984-98-5 ]
[ 1158984-98-5 ]
| Yield | Reaction Conditions | Operation in experiment |
| 98% |
|
Under ambient atmosphere, to a 40 mL I-Chem vial equipped with a stir bar was added 1-te/t-butoxy-4-chlorobenzene (3a) (0.185 g, 1.00 mmol), dicyclohexylphosphino^δ'-dimethoxy-I J '-biphenyl (SPhos) (41 mg, 0.10 mmol) and Pd(OAc)2 (11 mg, 0.050 mmol). The vial was sealed with a PTFE-lined septum screw- cap, and then placed under an Ar atmosphere. To the vial was added dioxane (9.5 mL) and the resulting mixture was stirred at 23 0C for 10 min. To the vial was added aqueous K3PO4 (3.0 M, 2.5 mL, degassed by sparging with Ar for 30 min). The vial was placed in a 60 0C oil bath, and to the stirred mixture was added dropwise over 3 h via syringe pump freshly prepared 2-furylboronic acid (1a) (0.1 12 g, 1.00 mmol) as a solution in dioxane (3.0 mL). After the addition was complete the reaction mixture was stirred at 60 0C for an additional 3 h. The mixture was cooled to room temperature and was then transferred to a 60 mL separatory funnel and was diluted with aqueous NaOH (1.0 M, 10 mL). The mixture was extracted with Et2θ (3 x 10 mL). The combined organic fractions were dried over MgSO4, filtered and then concentrated in vacuo. The resulting residue was purified by flash chromatography (hexanes: EtOAc, 100:0 -> 9:1) to afford a colorless oil (0.213 g, 98%). |
- 13
-
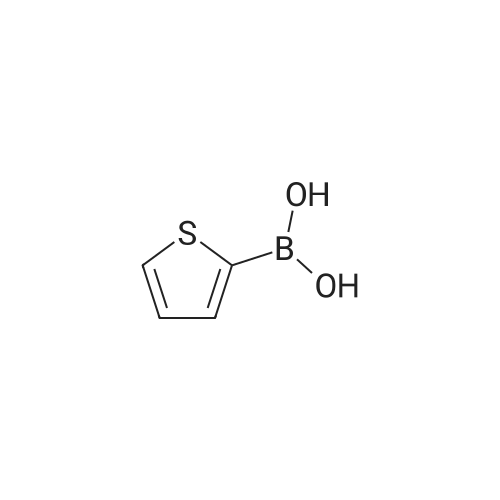 [ 6165-68-0 ]
[ 6165-68-0 ]

-
 [ 18995-35-2 ]
[ 18995-35-2 ]

-
 [ 808142-44-1 ]
[ 808142-44-1 ]
- 14
-
 [ 1104636-73-8 ]
[ 1104636-73-8 ]

-
 [ 18995-35-2 ]
[ 18995-35-2 ]

-
4-tert-butoxystyrene
[ No CAS ]

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping