|
|
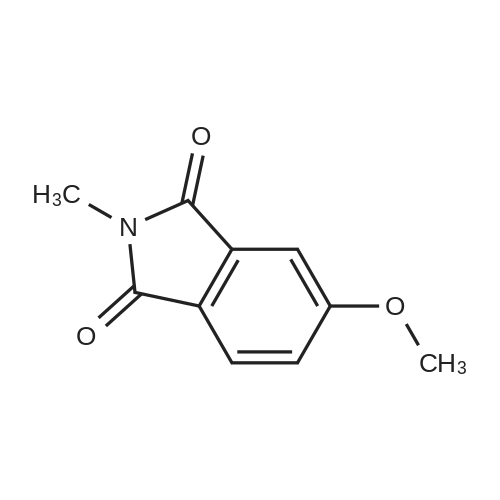
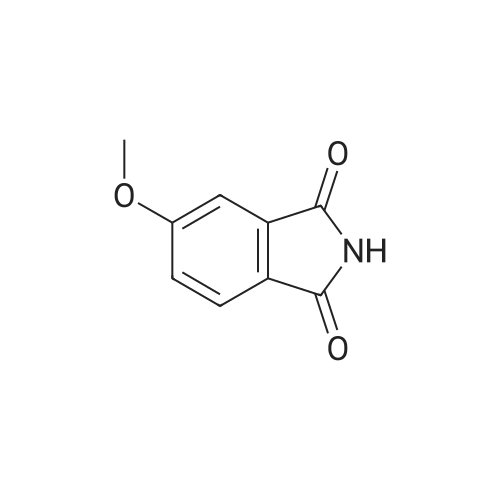
Example 3 5-(5-Methoxy-1,3-dioxo-1,3-dihydro-isoindol-2-ylmethyl)-2-(oxalyl-amino)-4,7-dihydro-5H-thieno[2,3-c]pyran-3-carboxylic acid; To a solution of 4-hydroxyphthalic acid (0.25 g, 1.37 mmol) in anhydrous N,N-dimethylformamide (3 ml) under nitrogen was added sodium hydride (0.22 g, 5.48 mmol). The solution was stirred for 5 minutes and then methyl iodide (0.68 ml) was added and continued stirring for 3 hours. Several drops of water were added to quench the reaction and the mixture was concentrated in vacuo. The crude material was partitioned between ethyl acetate (40 ml) and water (10 ml). The layers were separated and the organic layer washed with brine (2×10 ml), dried (Na2SO4), filtered and the solvent evaporated in vacuo. The resulting oil was dissolved in methanol (8 ml) and 1N sodium hydroxide (4 ml) was added. The reaction was stirred at ambient temperature for 24 h., after which LC-MS indicated only partial hydrolysis. The material was reconstituted in methanol (5 ml) and treated with of sodium hydroxide (0.12 g, 3.0 mmol) dissolved in water (1 ml). The reaction mixture was stirred for 48 h., at which time a precipitate had formed. The mixture was acidified with 6N hydrochloric acid until pH=1, causing the solution to become homogeneous. The reaction was concentrated in vacuo and the residue partitioned between ethyl acetate (30 ml) and 0.5N hydrochloric acid (10 ml). The layers were separated and the organic layer concentrated in vacuo to give 100 mg (51%) of 4-methoxy-phthalic acid as a solid.1H NMR (300 MHz, CD3OD) delta 7.83 (d; J=8, 1H), 7.10-7.06 (m, 2H), 3.87 (s, 3H).LC-MS: Rt=1.45 min, [M+H]+=197.1A solution of 4-methoxy-phthalic acid (0.10 g, 0.51 mmol), 1-hydroxy-benzotriazole (0.15 g, 1.1 mmol), 1-(3-dimethylaminopropyl)-3-ethyl-carbodiimide hydrochloride (0.22 g, 1.1 mmol), and triethylamine (0.35 ml, 2.5 mmol) was prepared in distilled acetonitrile (4 ml) under nitrogen. 2-Amino-5-aminomethyl-4,7-dihydro-5H-thieno-[2,3-c]pyran-3-carboxylic acid tert-butyl ester (0.11 g, 0.39 mmol) was added in small portions and the reaction was stirred at ambient temperature for 18 h., and then concentrated in vacuo. The crude mixture was diluted in ethyl acetate (30 ml) and washed with 1% hydrochloric acid (5 ml), saturated sodium bicarbonate (5 ml), and brine (5 ml). The organic layer was dried (Na2SO4), filtered, and the solvent evaporated in vacuo. The crude material was purified by silica gel chromatography using a 10% mixture of ethyl acetate/dichloromethane as eluant. Pure fractions were collected and the solvent evaporated in vacuo to give 54 mg (31%) of 2-amino-5-(5-methoxy-1,3-dioxo-1,3-dihydro-isoindol-2-ylmethyl)-4,7-dihydro-5H-thieno-[2,3-c]pyran-3-carboxylic acid tert-butyl ester.1H NMR (300 MHz, CDCl3) delta 7.76 (d, J=8, 1H), 7.32 (s, 1H), 7.14 (d, J=8, 1H), 4.62-4.48 (m, 2H), 4.00-3.72 (m, 3H), 3.91 (s, 3H), 2.86 (d, J=17, 1H), 2.55 (dd, J=17, 10, 1H), 1.49 (s, 9H).To a solution of the above 2-amino-5-(5-methoxy-1,3-dioxo-1,3-dihydro-isoindol-2-ylmethyl)-4,7-dihydro-5H-thieno-[2,3-c]pyran-3-carboxylic acid tert-butyl ester (54 mg, 0.12 mmol) in distilled dichloromethane (3 ml) under nitrogen was added midazol-1-yl-oxo-acetic acid tert-butyl ester (0.25 g, 0.36 mmol) and triethylamine (50 mul, 0.36 mmol). The reaction was stirred for 4 h., concentrated in vacuo and the residue reconstituted in ethyl acetate (20 ml). The organic layer was washed with 1% hydrochloric acid (2×5 ml), saturated sodium bicarbonate (5 ml), and brine (5 ml). The organic phase was dried (Na2SO4), filtered, and the solvent evaporated in vacuo. The crude material was purified by silica gel chromatography using a 5% mixture of ethyl acetate/dichloromethane as eluant. Pure fractions were collected and the solvent evaporated in vacuo to give 56 mg (81%) of 2-(tert-butoxyoxalyl-amino)-5-(5-methoxy-1,3-dioxo-1,3-dihydro-isoindol-2-ylmethyl)-4,7-dihydro-5H-thieno-[2,3-c]pyran-3-carboxylic acid tert-butyl ester.1H NMR (300 MHz, CDCl3) delta 12.48 (s, 1H), 7.75 (d, J=8, 1H), 7.32 (d, J=2, 1H), 7.15 (dd, J=8, 2, 1H), 4.78 (d, J=15, 1H), 4.65 (d, J=15, 1H), 4.03-3.75 (m, 3H), 3.91 (s, 3H), 2.95 (d, J=17, 1H), 2.66 (dd, J=17, 9, 1H), 1.58 (s, 9H), 1.54 (s, 9H).APCI-MS: [M+H]+=574The above 2-(tert-butoxyoxalyl-amino)-5-(5-methoxy-1,3-dioxo-1,3-dihydro-isoindol-2-ylmethyl)-4,7-dihydro-5H-thieno-[2,3-c]pyran-3-carboxylic acid tert-butyl ester (55 mg, 0.096 mmol) was dissolved in a solution of 50% trifluoroacetic acid/dichloromethane (4 ml). The reaction was stirred at ambient temperature for 7 h., concentrated in vacuo and evaporated in vacuo from dichloromethane (3×10 ml). The resulting precipitate was washed with dichloromethane and dried in vacuo to give 17 mg (40%) of the title compound as a solid.1H NMR (300 MHz, DMSO-d6) delta 12.32 (s, 1H), 7.81 (d, J=8, 1H), 7.40 (d, J=2, 1H), 7.31 (dd, J=8, 2, 1H), 4.75 (d, J=15, 1H), 4.56 (d, J=15, 1H), 3.92 (s, 3H), 3.91-3.69 (m, 3H), 2.98 (d, J=17, 1H), 2.57 (dd, J=17, 9, 1H).AP... |
|
With water; sodium hydroxide; In acetone; at 20℃; |
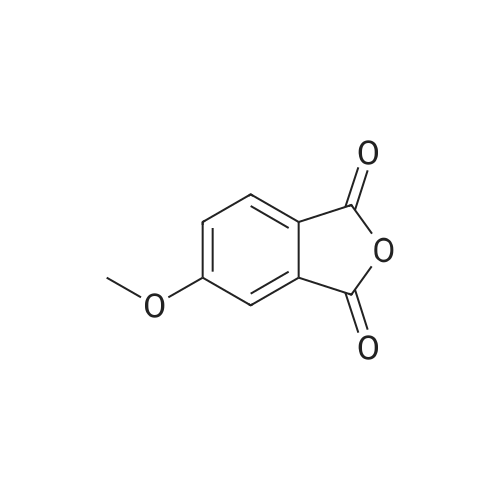
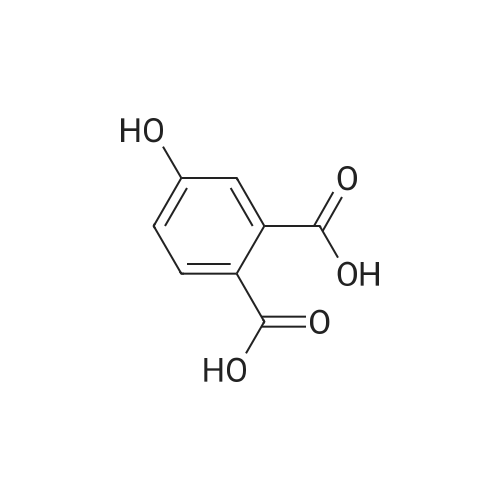
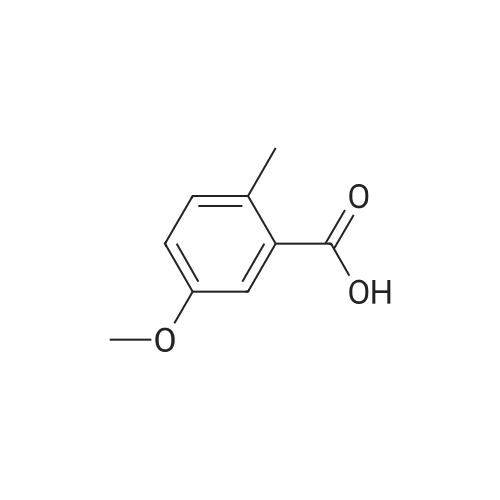
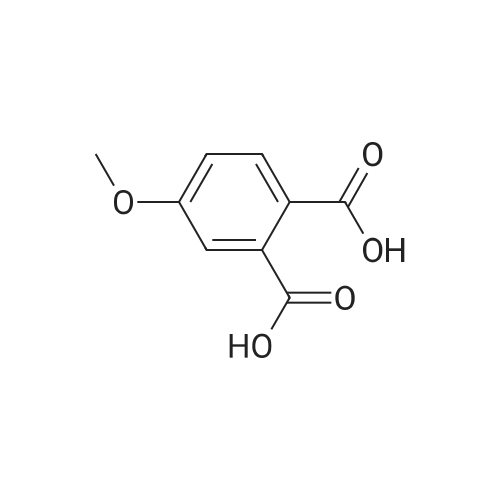
The 3 ml of acetone containing the compound prepared in step 2 (0.31 g, 1.4 mmol) dissolved therein was treated with 2 ml of water containing NaOH (0.34 g, 8.4 mmol) dissolved therein, followed by stirring at room temperature for overnight. After evaporating acetone, the reaction mixture was acidized with 6 M HCl to adjust to pH 2, followed by extraction with ethyl acetate. The combined organic layer was dried over MgSO4, and the solvent was eliminated under reduced pressure. As a result, a crude target compound was obtained (0.27 g, 97%). 1H NMR (DMSO-d6, 500 MHz) delta 12.94 (s, 2H), 7.74 (d, J=8.5 Hz, 1H), 7.07 (dd, J=8.5, 2.7 Hz, 1H), 7.05 (d, J=2.5 Hz, 1H), 3.83 (s, 3H). 13C NMR (DMSO-d6, 125 MHz) delta 169.3, 167.4, 161.4, 137.1, 131.3, 122.7, 115.1, 113.0, 55.7. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping