| 49% |
|
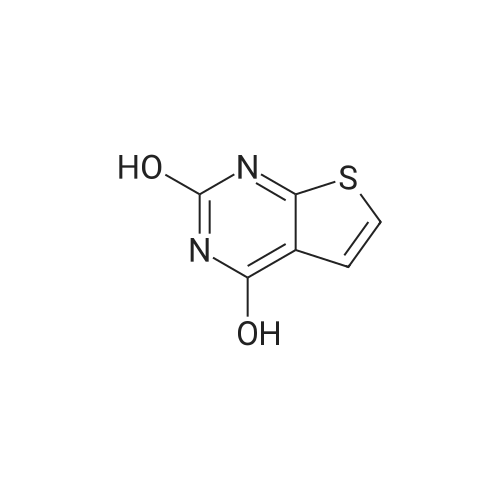
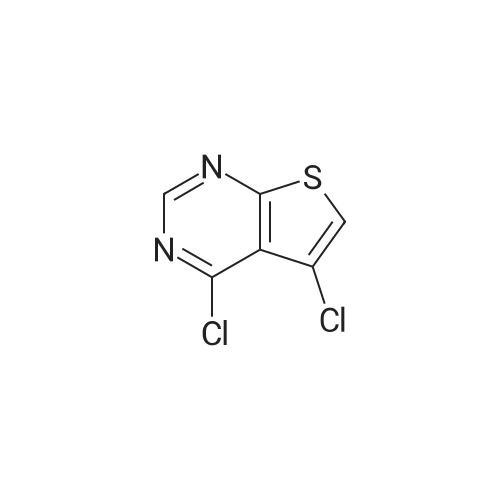
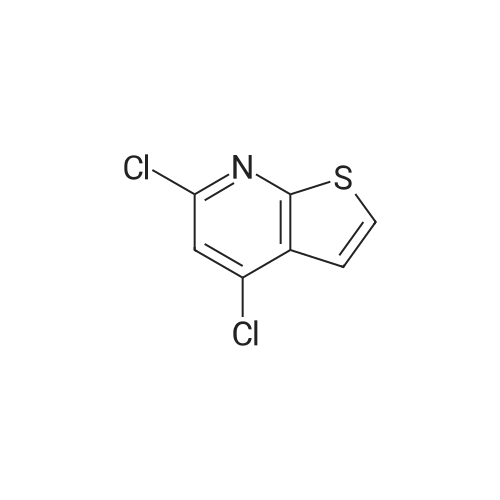
3 g (0.018 mol) of the intermediate lib was placed in a 100 mL three-necked flask,30 mL (0.329 mol) of phosphorus oxychloride was slowly added dropwise,After 30 min addition, 2 drops of DMF were added to the reaction mixture and the reaction was heated to reflux for 8 h. The reaction was poured into 1000 g of ice water with stirring, and a large amount of orange-colored solid was precipitated. The filter cake was washed with a large amount of water and dried at 45 C for 24 hours to obtain 1.8 g of an orange solid in a yield of 49%. |
| 41% |
With trichlorophosphate; at 106℃; for 3h;Inert atmosphere; |
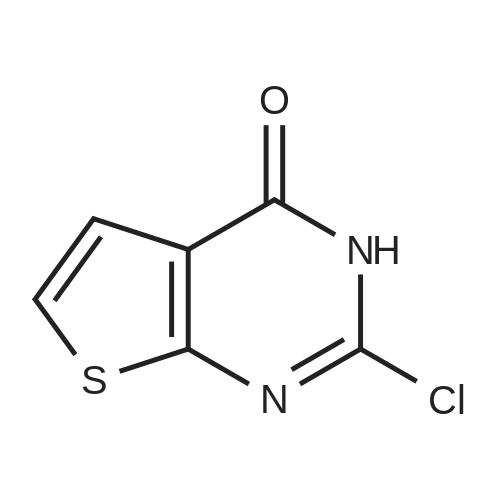
Under a nitrogen streamThieno [2,3-d] pyrimidine-2,4 (1H, 3H) -dione (8.4 g, 50.0 mmol) and phosphoryl chlorideL), which was stirred for 3 hours at 106 C. After completion of the reaction, the organic layer was separated using ethyl acetateWater was removed using MgSO4. The solvent of the organic layer was removed, and the residue was purified by column chromatography to obtain the target compound2,4-dichlorothieno [2,3-d] pyrimidine (4.2 g, 20.5 mmol, yield 41%). |
| 40% |
With trichlorophosphate; at 116℃; for 3h; |
A mixture of thieno[2,3-phiyrimidine-2,4(l//,3//)-dione (100 mg, 0.59 mmol) and phosphonyl chloride (2 mL, 21.5 mmol) was heated at 1 16 0C for 3 h. Upon completion of the reaction, the reaction mixture was poured into ice and extract with ethyl acetate 3 times. The combined organic layers were washed with brine, dried over Na2SO4, filtered and concentrated in vacuo. Purification by column chromatography, eluting with Hexanes/Ethyl Acetate (9: 1) afforded the product as a white solid (48.1 mg, 40%): 1H NMR (DMSO, 300 MHz) delta 7.6 (d, J = 6.3 Hz, IH), 8.13(d, J = 5.7 Hz, IH). |
| 35.5% |
With N,N-dimethyl-aniline; trichlorophosphate; for 16h;Reflux; |
A total of 0.8 mL N,N-dimethylaniline was added to 3.0 g of thieno[2,3-d]pyrimidine-2,4-(1H,3H)-dione 6a in 20 mL POCl3. The mixture was then heated under reflux for 16 h. Excess POCl3 was removed in vacuo, and the resulting residue was treated with ice water to yield a precipitate. The solid was collected by filtration, washed with water and dried over a funnel to afford solid 7a (1.3 g, yield 35.5%). 1H NMR (400 MHz, CDCl3): delta 7.62 (d, J = 6.4 Hz, 1H), 7.43 (d, J = 6.4 Hz, 1H). |
|
With trichlorophosphate; at 200℃; for 2.3h; |
EXAMPLE 402,4-Dichlorothieno[2,3-d]pyrimidine; The dione of Example 39 (2.6 g) was placed into a pressure vessel with phosphorus oxychloride (15 mL). The mixture was heated at 200 0C for 2.3 hours and then cooled to room temperature and concentrated under reduced pressure. Residual phosphorus oxychloride was azeotroped . twice with toluene (30 mL) under reduced pressure. The residue was partitioned between saturated aqueous sodium bicarbonate and dichloromethane. The resulting layers were separated and the organic layer was filtered through anhydrous magnesium sulfate and concentrated to dryness under reduced pressure to give 1.06 g of the title compound: MS (ESI+) for C6H2N2CI2S m/z205.0 (M+H)+. 1H NMR (400 MHz, CDCI3) delta 7.42 (d,1 H), 7.61 (d,1 H). |
|
With N,N-dimethyl-aniline; trichlorophosphate; In toluene; at 100℃; for 3h; |
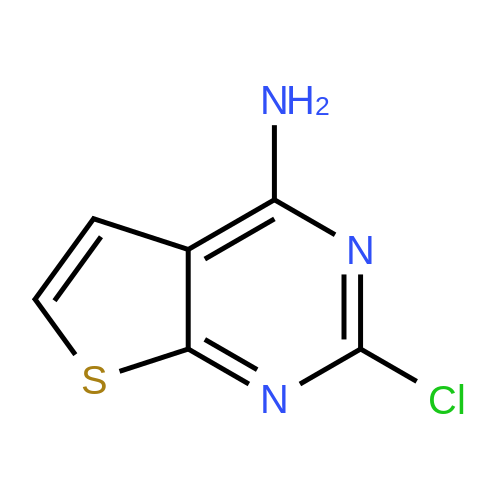
To a solution of 1H-thieno[2,3-d]pyrimidine-2,4-dione (62) (92.8 mg, 0.552 mmol) in toluene (1 mL) were added N,N-dimethylaniline (0.140 mL, 1.10 mmol) and phosphoryl chloride (0.280 mL, 3.00 mmol). The reaction mixture was warmed to 100 C and stirred for 3 h. After cooling to room temperature, H2O was added to the reaction mixture and the mixture was extracted with CHCl3. The combined organic layer was washed with brine, dried over Na2SO4, and concentrated in vacuo to give 2,4-dichlorothieno[2,3-d]pyrimidine (63). This compound was used for the next reaction without further purification.To a solution of 63 in DMF (4 mL) was added ethyl 2-(4-aminophenyl)acetate (95.2 mg, 0.531 mmol) and the reaction mixture was stirred at 65 C for 2.5 h. After cooling, H2O was added to the reaction mixture and the mixture was extracted with EtOAc. The combined organic layer was washed with brine, dried over Na2SO4, and concentrated in vacuo. The residue was chromatographed (EtOAc/hexane = 0% to 100%) to give the title compound (85.9 mg, 0.247 mmol, 45%) as a colorless solid. 1H NMR (CDCl 3) delta: 1.28 (3H, t, J = 7.4 Hz), 3.64 (2H, s), 4.18 (2H, q, J = 7.4 Hz), 7.03 (1H, d, J = 5.7 Hz), 7.21 (1H, s), 7.29 (1H, d, J = 5.7 Hz), 7.32 (2H, d, J = 8.6 Hz), 7.56 (2H, d, J = 8.6 Hz). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping