| 76% |
In tert-butyl methyl ether; at 20℃; for 18.0h; |
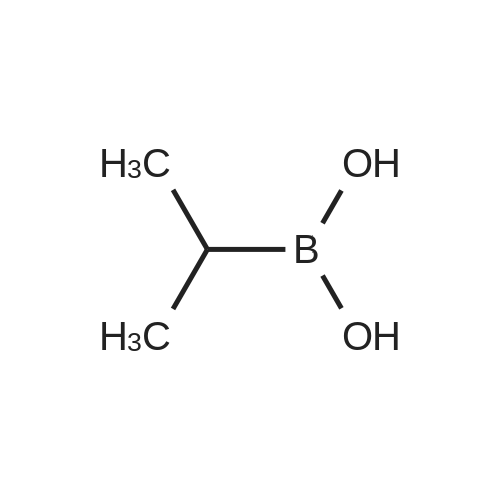
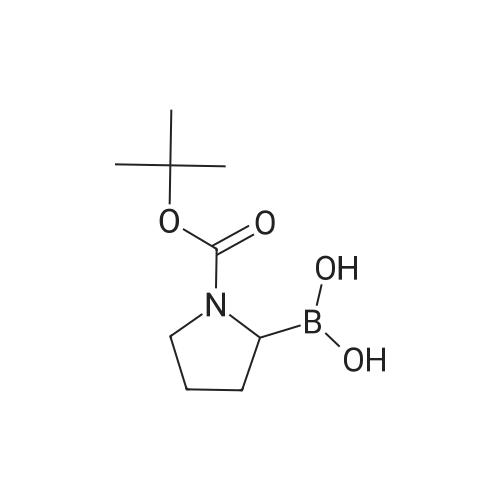
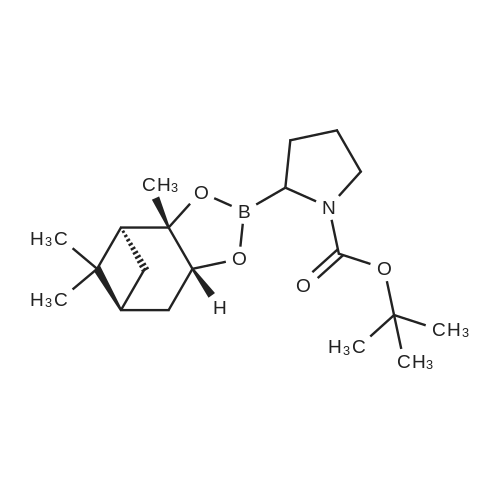
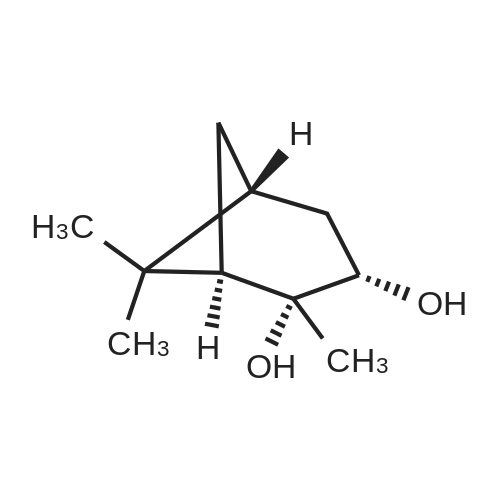
Example 1; Synthesis of (2R)-boroPro- (lS, 2S, 3R, 5S)-pinanediol ester, hydrochloride (2); [0280] A flame dried round bottom flask equipped with a magnetic stir bar was charged with N-Boc-pyrrolidine (20 g, 117 mmol, 1 eq) and dry THF (60 mL) under a nitrogen atmosphere. The clear colorless solution was cooled to-78C and a solution of s- BuLi (100 mL of a 1.4 M solution in cyclohexane, 140 mmol) was added slowly over a 30 minute period. The light orange colored solution was stirred at-78C for 3 hours followed by treatment with B (OMe) 3 (39 mL, 350 mmol) after which the cooling bath was removed and the clear colorless solution slowly warmed to 0C. Upon reaching 0C, the reaction was quenched with a small amount of water (-2 mL), allowed to warm to room temp then extracted into 2 N NaOH (250 mL) and backwashed with additional EtOAc (150 mL). The aqueous phase was acidified to pH 3 by the addition of 2 N HCl and then extracted with EtOAc (3 x 120 mL). The organic extracts were combined and dried over Na2SO4 and concentrated to produce the free boronic acid (22.08 g, 103 mmol) as a sticky white solid in 88% yield. Without further purification the boronic acid was dissolved in tert-butyl methyl ether (150 mL) and with constant stirring (+) -pinanediol (17.5 g, 103 mmol) was added at room temperature. After 18 hr the ether was removed and the (+) -pinanediol boronic ester was purified by column chromatography (silica gel, 1: 3 hexanes/EtOAc) to give a clear thick oil (26.84 g, 76.8 mmol, 76% yield, Rf= 0.6 using a 2: 1 hexane/ethyl acetate eluant, made visual via 12 and/or PMA stain). Removal of the Boc protecting group was achieved by dissolving the oil in dry ether, cooling to 0C in an ice bath and with constant stirring dry HCl (g) was bubbled into the solution for 10 minutes. After 2 hours a white precipitate developed in the flask and the ether and excess HCl were removed in vacuo to afford the racemic HCl salt as a white solid. Crystallization and isolation of the desired isomer was performed by dissolving the HCI salt in a minimal amount of dichloromethane (250 mL) with gentle heating to facilitate a homogenous solution followed by continuous stirring for 8 hours to yield a fluffy white precipitate that was collected by vacuum filtration, dried and then dissolved in minimal 2-propanol (-200 mL) with gentle heating until homogenous. The alcoholic solution was stirred over night and the resulting white precipitate was collected by vacuum filtration affording isomerically pure 1 as a white solid. (7.0 g, 27 mmol, 23% yield).'H NMR (400 MHz, D20) 8 4.28 (d, J= 8.0 Hz, lH), 3.06 (m, 3H), 2.18 (m, 1H), 1.96 (m, 2H), 1.78 (m, 3 H), 1.62 (m, 2H), 1.21 (s, 3H), 1.05 (m, 5H), 0.84 (d, J=12 Hz, 2H), 0.71 (s, 2H), 0.62 (s, 3H). |
| 12.1% |
In ethyl acetate; at 20℃; for 18.0h; |
General procedure: A flame dried round bottom flask equipped with a magnetic stir bar was charged with N-Boc-pyrrolidine (10g, 58mmol, 1eq) and dry THF (40mL) under a nitrogen atmosphere. The clear colorless solution was cooled to -78C and a solution of s-BuLi (64mL of a 1.0M solution in cyclohexane, 64mmol) was added slowly over a 30min period. The light orange colored solution was stirred at -78C for 3h followed by treatment with B(OMe)3 (15mL, 175mmol) after which the cooling bath was removed and the clear colorless solution slowly warmed to 0C. Upon reaching 0C, the reaction was quenched with a small amount of water (?2mL), allowed to warm to room temp then extracted into 2N NaOH (100mL) and backwashed with additional EtOAc (60mL). The aqueous phase was acidified to pH 3 by the addition of 2N HCl and then extracted with EtOAc (3×60mL). The organic extracts were combined and dried over Na2SO4 and concentrated to produce the free boronic acid 9g as a sticky white solid. Without further purification the boronic acid was dissolved in EtOAc (60mL) and with constant stirring (+)-pinanediol (7.0g, 41mmol) was added at room temperature. After 18h the ester was removed and the (+)-pinanediol boronic ester was purified by column chromatography (silica gel, 6:1 hexanes/EtOAc) to give a clear thick oil (12.1g, 34.8mmol) 60% yield in two steps. 1H NMR (400MHz, CDCl3) delta 4.50-4.15 (m, 1H), 3.38 (dt, J=13.8, 6.1Hz, 2H), 3.12 (ddd, J=25.1, 15.8, 8.4Hz, 1H), 2.33 (dd, J=12.3, 10.3Hz, 1H), 2.20 (s, 1H), 2.10-1.69 (m, 7H), 1.45 (d, J=7.3Hz, 9H), 1.41 (s, 3H), 1.28 (s, 3H), 0.84 (s, 3H). |
| 2.52 g |
In tert-butyl methyl ether; at 20℃; for 12.0h; |
Free boric acid C1 of Example 1 (2.1 g, 9.36 mmol)Soluble in 20mL methyl tert-butyl ether,Add (+)-pinanediol (1.75 g, 10 mmol) at room temperatureStirring was continued, and the reaction was completed after 12 hours.The solvent was distilled off and directly subjected to column chromatography (petroleum ether: ethyl acetate = 10:1)A clear viscous oil of 2.52 g was obtained in a yield of 76%. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping