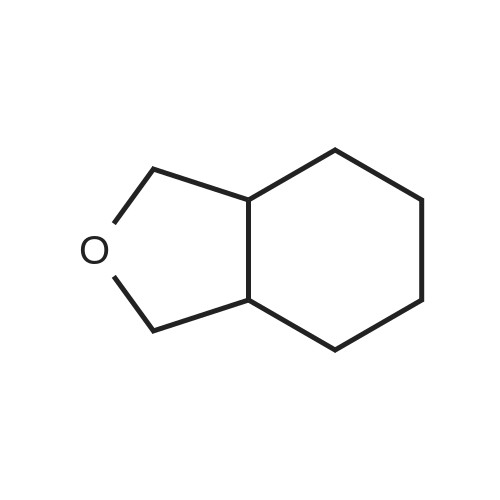
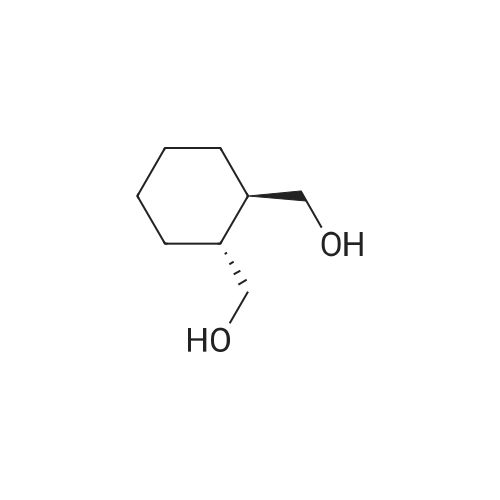
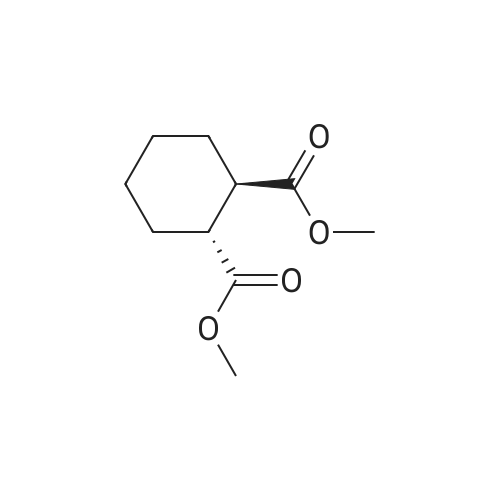
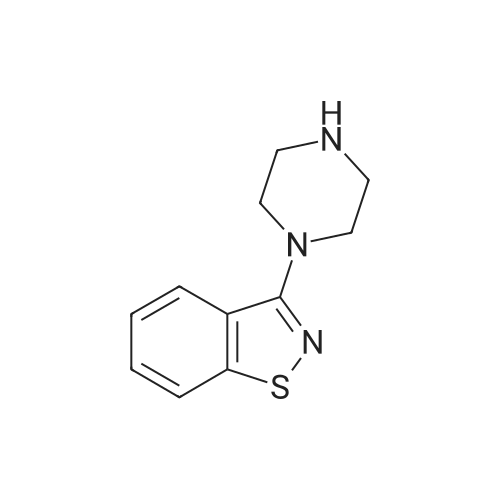
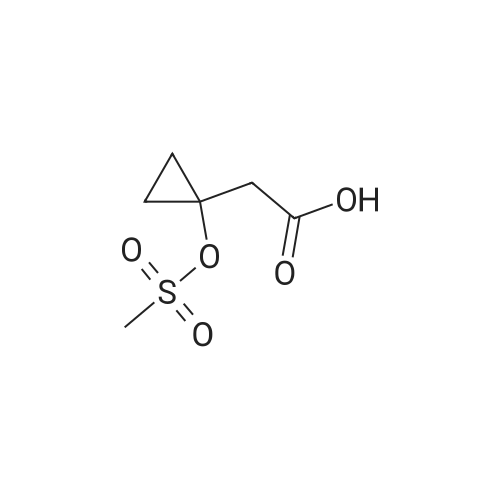
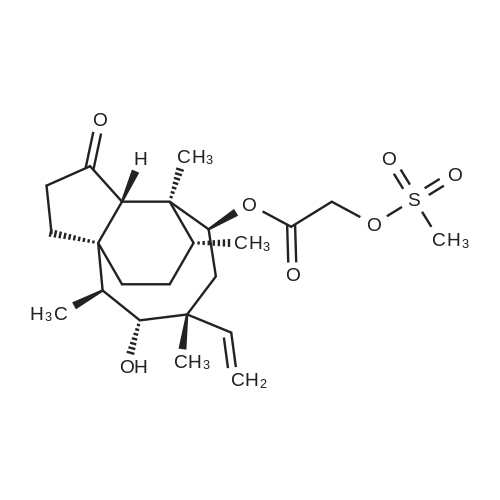
| 88% |
With sodium carbonate; In acetonitrile; for 30h;Reflux; |
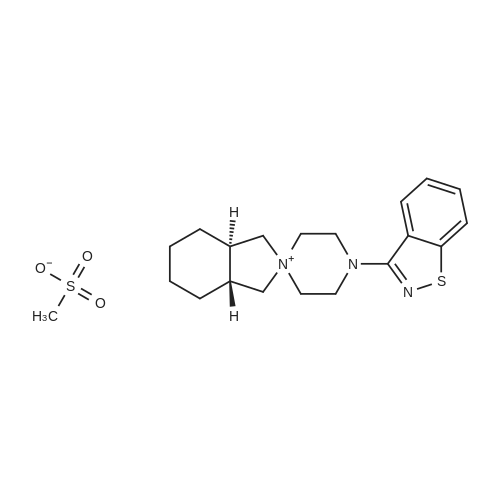
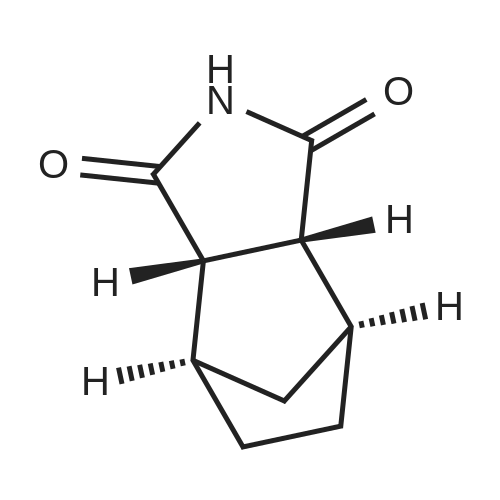
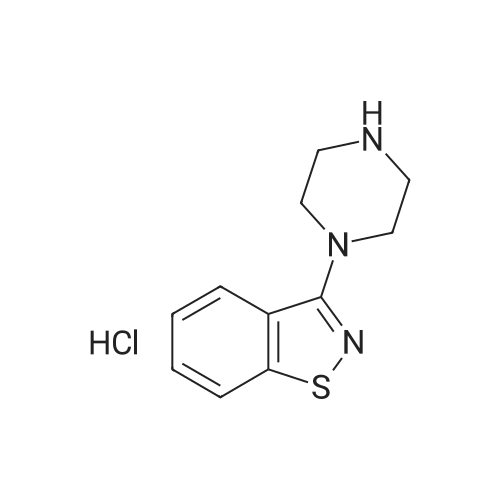
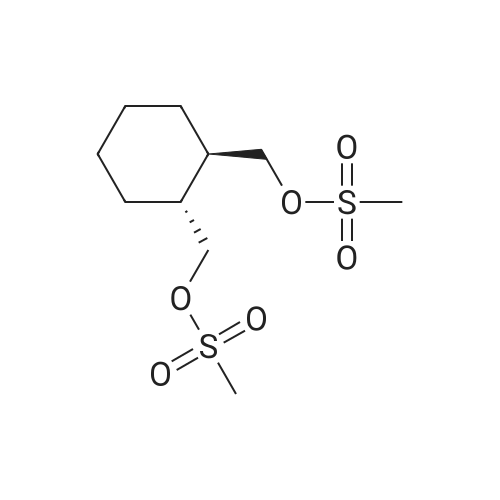
3-(l-Piperazinyl-l,2-benzisothiazole) (13.2 g) and sodium carbonate (6.5 g) were added to a solution of trans (R,R)-l,2-bis(methanesulfonylmethyl)cyclohexane (18 g) in acetonitrile (180 mL) at ambient temperature. The reaction mixture was refluxed for about 30 hours, filtered and washed with acetonitrile (2x25 mL). The combined filtrate was concentrated at about 60C under reduced pressure. Acetone (40 mL) was added to the residue and the reaction mixture was stirred at about 40C until the product precipitated out. Hexane (50 mL) was added. The reaction mixture was stirred for about 30 minutes at ambient temperature, filtered and dried under reduced pressure at about 45C for about 8 hours to obtain trans (R,R)-3a,7a-octahydroisoindolium-2-spiro-l '-[4'- (l,2-benzoisothiazole-3-yl)]piperazine methane sulfonate.Yield: 88% |
| 80% |
With potassium carbonate; In acetonitrile; for 20h;Reflux; |
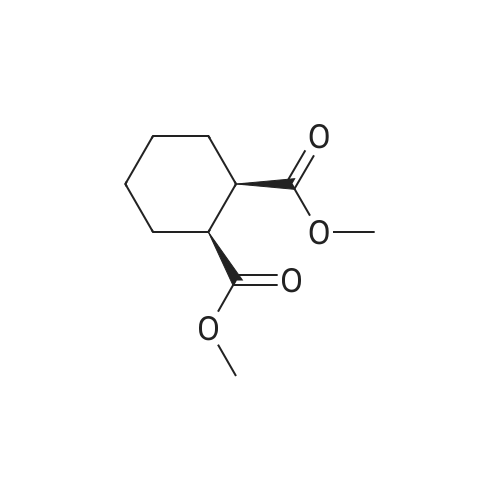
A mixture of (1R,2R)-cyclohexane-1,2-diyldimethanediyl dimethanesulfonate (1) (11.7 g, 38.9 mmol), 3-(piperazin-1-yl)-1,2-benzisothiazole (2) (7.76 g, 35.4 mmol), potassium carbonate (4.9 g, 35.4 mmol) and acetonitrile (200 ml) was refluxed for 20 hours. The mixture was filtrated at the hot state thereof, and the filtrate was concentrated to give Compound (3) (12 g, 28.3 mmol, yield: 80%). |
|
With dipotassium hydrogenphosphate; tetra(n-butyl)ammonium hydrogensulfate; In water; toluene; for 15h;Reflux;Product distribution / selectivity; |
Example 1To a mixed solution of 4-(1,2-benzisothiazol-3-yl)piperazine [Compound (A)] (20.0 g, 91.2 mmol), <strong>[186204-35-3](1R,2R)-1,2-bis(methanesulfonyloxymethyl)cyclohexane</strong> [Compound (B)] (32.9 g, 109.5 mmol), and toluene (280 g) were added dibasic potassium phosphate (47.7 g, 273.9 mmol), water (1.4 g, 77.8 mmol) and tetra-n-butyl ammonium hydrogen sulfate (1.2 g, 3.5 mmol). The mixture was stirred under reflux for 15 hours (water (0.5 g) was added in mid-course) to give a reaction mixture containing 4'-(1,2-benzisothiazol-3-yl)-(3aR,7aR)-octahydro-spiro[2H-isoindole-2,1'-piperazinium]methanesulfonate [Compound (C)]. |
|
In toluene; for 3h;Reflux;Product distribution / selectivity; |
Example 1A mixed solution of 4-(1,2-benzisothiazol-3-yl)piperazine [Compound (A)] (20.0 g, 91.2 mmol), <strong>[186204-35-3](1R,2R)-1,2-bis(methanesulfonyloxymethyl)cyclohexane</strong> [Compound (B)] (13.7 g, 45.6 mmol), and toluene (140 g) was stirred under reflux for 3 hours to give a reaction mixture containing 4'-(1,2-benzisothiazol-3-yl)-(3aR,7aR)-octahydro-spiro[2H-isoindole-2,1'-piperazinium]methanesulfonate [Compound (C)]. And, the production rate of by-product (R) was 0.025% (which was calculated with the following formula (a)). |
|
With tetra(n-butyl)ammonium hydrogensulfate; sodium carbonate; In acetonitrile; at 80 - 85℃; for 24h; |
100 g ( lR,2/?)-(-)-trans-cyclohexane- 1 ,2-diylbis(methylene)dimethanesulfonate of Formula (B) and 70 g 3-(piperazin-l-yl)benzo[d]isothiazole of Formula (F), 1500 mL acetonitrile and 35 g sodium carbonate and 0.975..g tetrabutyl ammonium hydrogen sulfate were added to round bottom flask at 25C to 35C and stirred for 10 min. The reaction mixture was heated to 80 to 85C for 24 hours. 35 g sodium carbonate and 0.975 g tetrabutyl ammonium hydrogen sulfate and stirred for 21 hours at 80 to 85C. After completion of the reaction, the reaction mixture was filtered and washed with acetonitrile. The wet-cake was heated with 300 mL acetonitrile at 80 to 85C and charcoalized. The reaction mixture was filtered and washed with acetonitrile. The filtrate was distilled under vacuum to remove acetonitrile. The residue was treated with 800 mL acetone and heated to 60C for 1 hour followed by cooling. The precipitated product was stirred for 2 hours at 25C and filtered. The wet-cake was recrystallized in 50 mL acetone at 60C to obtain titled compound (3aR,7aR)-4'-(benzo[d]isothiazol-3- yl)octahydrospiro[isoindole-2,l'-piperazin]-r-ium mesylate of Formula (G). X-ray powder diffraction pattern (FIG.5), DSC (FIG.6). |
|
With 1,8-diazabicyclo[5.4.0]undec-7-ene; In 1,4-dioxane; dimethyl sulfoxide; at 106℃; for 11h; |
Example 2 Synthesis of Lurasidone Base 480 g of compound 4 and 400 g of compound 5 are added to a mixture consisting of dioxane (960 mL) and dimethyl sulphoxide (48 mL). The mixture is heated to the reflux temperature of 106 C., and 560 g of DBU is dripped into it in 60 minutes. After ten hours' heating, 280 g of compound 2 and 560 g of DBU are added to the solution of compound 3 thus obtained and heated to 125-130 C., distilling about 300 mL of solvent. After ten hours' heating at said temperature 40 mg of DBU is added, and heating continues for a further 12 h. The mixture is then cooled to ambient temperature, diluted with 14 L of an acetone/water 1:2 mixture, and the lurasidone base (450 g) is filtered and dried. |
| 12.5 g |
With sodium carbonate; In acetonitrile; for 20h;Reflux; |
To a suspension of iran5(R,R)-l,2-bis(methanesulfonylmethyl)cyclohexane (15 g) in acetonitrile (150 mL) l-(l,2-benzisothiazol-3-yl)piperazine (10.95g) and sodium carbonate (7.8 g) were added, heated and stirred for 20 hrs at reflux temperature. Reaction was monitored by HPLC. After the completion of reaction, mass was cooled to 40-45 C, filtered and washed with acetonitrile (20 mL). The acetonitrile was distilled off under vacuum at 45-50 C. To the residue acetone (100 mL) was added, stirred for 1 hour, filtered, washed with acetone (10 mL), dried at 50-55C for 6-8 hours to get the product (12.5 g). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping