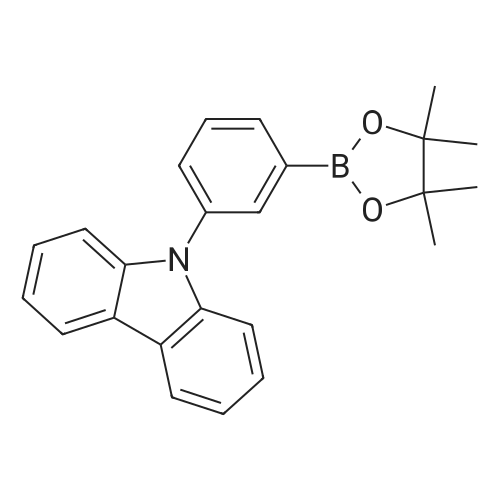
| 76% |
With dicyclohexyl-(2',6'-dimethoxybiphenyl-2-yl)-phosphane; tris-(dibenzylideneacetone)dipalladium(0); potassium acetate; In 1,4-dioxane; for 24h;Inert atmosphere; Reflux; |
In 500 mL three-necked flask, followed by adding intermediate M7 (20g, 62.lmmol), bis (pinacolato) diboron (23.6g, 93.2mmol), CH3COOK (17.5g, 178.2mmol), 1, 4- dioxane, 300 mL, and S-phos (5.35g, 13.04mmol), N2 replaced three times, was added Pd2 (dba) 3 (3.98g, 4.35mmol) and heated to reflux. After 24h the reaction was stopped.It cooled to room temperature, filtered, and the filtrate was collected, concentrated, and purified by column chromatography (dichloromethane: n-hexane = 0-1 as the mobile phase), to receive an off-white solid 17.4g (76%), |
| 70% |
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In tetrahydrofuran; 1,4-dioxane; at 85℃; for 24h; |
In a 250 mL single-mouth reaction flask, Compound 5 (5.00 g, 15.52 mmol) was added separately.Potassium acetate (7.90 g, 80.70 mmol) and bis(pinacol) diboron (4.88 g, 18.62 mmol), Pd(dppf) 2Cl2 (0.23 g, 0.31 mmol),Then, 100 mL of anhydrous tetrahydrofuran was added, followed by heating to 85 C under nitrogen for 24 hours.After completion of the reaction, it was cooled to room temperature, then 100 mL of water was added, extracted with dichloromethane, and the organic phase was collected and washed with water several times.The organic phase was dried over anhydrous magnesium sulfate and the solvent was evaporated with stirring.The column chromatography eluent was ethyl acetate/petroleum ether = 10/1, yielding a white solid, 4.2 g, yield 70%. |
| 69% |
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In N,N-dimethyl-formamide; at 85℃;Inert atmosphere; |
A mixture of 1B (6.55 g, 20.4 mmol), bis(pinacolato)diboron (6.22 g, 24.5 mmol), [1,1?-Bis(diphenylphosphino)ferrocene]dichloropalladium (747 mg, 1.02 mmol), and potassium acetate (5.88 g, 60 mmol) in dimethylformamide (DMF) (anhydrous, 50 mL) was degassed for 45 minutes. The mixture was further degassed by being heated to about 85 C. overnight under argon. After cooling to RT, the mixture was poured into DCM (250 mL) and solids were filtered off. The filtrate was washed with water (2×300 mL), dried over sodium sulfate, and loaded onto silica gel. Flash column (gradient of 10% to 30% DCM in hexanes) and precipitation from DCM/MeOH gave 5.17 g of 1C; 69% yield, pure by HNMR. |
| 68% |
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In 1,4-dioxane; at 80℃; for 24h;Inert atmosphere; |
Step 1 in the manufacturing of the compound (3.0 g, 9.3 mmol), bis (pinacolato) di boron (2.6 g, 10 mmol), potassium acetate (2.7 g, 28 mmol) and [bis (diphenylphosphino)ferrocene] dichloropalladium (0) complex (0.32 g, 0.39 mmol) and then the presence of nitrogen, for 24 hours at 80 dissolved in dioxane (60 ml), and stirred. After the reaction was terminated, cooled to ambient temperature, the reaction mixture was filtered through celite. Pour water to the filtrate, extracted with ethyl acetate, and wash the organic layer extracted with brine. The washed organic layer was dried over magnesium sulfate and purified by column chromatography (silica gel), the desired compound (2.3 g, 68%) was obtained. |
| 68% |
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; at 100℃; for 26h;Inert atmosphere; |
4 was prepared as follows: a mixture of compound 3 (see Kido, J.; Su, S. -J.; Sasabe, H.; and, Takeda, T., Chem. Mater. 2008, 20(5), 1691-1693, which is incorporated by reference herein for its relevant teachings) (5.45 g, 16.9 mmol), bis(pinacolato)diboron (9.020 g, 35.52 mmol), [1,1'-bis(diphenylphosphino)-ferrocene]dichloropalladium(II) (0.743 g, 1.02 mmol), potassium acetate (4.980, 50.75 mmol) and anhydrous toluene (110 mL) was degassed with argon for 1 h. while stirring. The reaction mixture was then maintained under argon at 100 C. while stirring for 25 h. until TLC (SiO2, 4:1 hexanes-dichloromethane) confirmed consumption of the starting material. Upon completion, the reaction was cooled to RT and about 500 mL ethyl acetate added. The organics were then washed with saturated NaHCO3, H2O and brine, dried over MgSO4, filtered and concentrated in vacuo. The crude product was purified via flash chromatography (SiO2, 4:1 hexanes-dichloromethane to 100% dichloromethane) to afford Compound 4 (4.26 g, 68%) as a white solid. |
|
With potassium acetate;1,1'-bis-(diphenylphosphino)ferrocene; tris-(dibenzylideneacetone)dipalladium(0); In 1,4-dioxane;Inert atmosphere; Reflux; |
Step 2. 3-Bromophenyl carbazole (9.5 g, 29.5 mmol), bis(pinacolato)diboron (11.2 g, 44.2 mmol), potassium acetate (8.7 g), tris(dibenzylideneacetone)dipalladium (200 mg) and 1,1'-bis(diphenylphosphino)ferrocene (400 mg) are suspended in 200 mL of dioxane and heated to reflux under nitrogen atmosphere overnight. After cooling down and evaporation the residue is subjected to column chromatography on silica gel, providing 9-(3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)-9H-carbazole b(6.0 g, colorless crystals). |
|
With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; potassium acetate; In N,N-dimethyl-formamide; for 6h;Reflux; |
9-(3-bromo-phenyl)-9H-carbazole (9-(3-bromophenyl)-9H-carbazole, 5g, 15.5mmol), bis(pinacolato)diboron (bis(pinacolato)diboron, 6.3g , 24.8mmol), KOAc 4.5g (46.5mmol), were dissolved Pd (dppf) Cl2CH2Cl2 1.13g (1.55mmol) in DMF100ml was refluxed for 6 hours. After cooling to room temperature after completion of the reaction was isolated by extraction with EA / H2O (Sat'd NaHCO3). The organic layer was dried over MgSO4 and evaporated under reduced pressure to remove the solvent. Separated by column chromatography to obtain the desired product compound 2-2. |
|
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In 1,4-dioxane; at 85℃; for 4h;Inert atmosphere; |
In a 500mL reaction flask, add intermediate M4 (11g, 34.45mmol), diboronic acid pinacol ester (13.12g, 51.68mmol), PdCl2 (dppf) (5.04g, 6.89mmol), CH3COOK (6.76g, 68.9mmol) Add 1,4-dioxane (10mL) and stir,Heated to 85 under nitrogen protection,After 4 hours of reaction, the reaction was monitored by TLC until the reaction was complete. The temperature was lowered to room temperature, filtered, and the reaction solution was concentrated.The product was separated and purified on a silica gel column, and concentrated to obtain a crude product, which was filtered with 100 mL of n-hexane to obtain intermediate M5. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping