|
In ethyl acetate; at 78℃; for 6h;Product distribution / selectivity; |
Example 1About 4 parts of ethyl acetate were charged into a reactor, under stirring. About 1.4 parts of MFA, about 0.045 parts of activated carbon and about 1 part of EPOC were added.The mixture was heated to the reflux temperature of ethyl acetate (about 780C).The reaction mixture was stirred at about 780C for about six hours.The progress of the reaction was checked by TLC.When the reaction was completed, the mixture was filtered at a temperature not below 650C in order to separate the activate carbon. EPO <DP n="7"/>The mixture was cooled to room temperature and then to about 0 ÷ -50C and stirred for about1 hour.The resultant crystals were separated by centrifugation and washed with about 1 part of ethyl acetate.The resultant wet crude carvedilol was charged into a stainless steel reactor and about 6 parts of ethyl acetate and 0.045 parts of activated carbon were added.The mixture was heated to the reflux temperature of ethyl acetate (about 780C), until the dissolution of the crystals. The mixture was stirred at about 780C for about 1 hour and then filtered at a temperature not below 650C in order to separate the activate carbon.The mixture was allowed to cool at about 2O0C and then to about 0 ÷ -50C and stirred for about 1 hour.The resultant crystals were separated by centrifugation and washed with about 1 part of ethyl acetate. The wet crystallized carvedilol was charged into a stainless steel reactor and about 4 parts of ethyl acetate were added.The mixture was heated to the reflux temperature of ethyl acetate (about 780C), until dissolution of the crystals.The mixture was stirred at about 780C for about 1 hour and filtered at a temperature not below 650C.The mixture was allowed to cool at about 2O0C and then to about 0 ÷ -50C and stirred for about 1 hour.The resultant crystals were separated by centrifugation and washed with about 1 part of ethyl acetate. The wet product was dried in an air dryer at 5O0C until the residual solvent ethyl acetate was within the specifications.Yield: about 1.05 to 1.10 parts of pure carvedilol for 1 part of EPOC.; Example 2 By repeating the procedure as described in example 1 but carrying out the reaction at a temperature of 7O0C, 6O0C and 5O0C, substantially the same results were obtained with a EPO <DP n="8"/>prolonged reaction time of 8 hours, 10 hours and 16.5 hours, respectively.; Example 3 The procedure as described in example 1 was repeated obtaining substantially similar results by using a molar ratio EPOGMFA of 1 : 1.5, 1 : 1.7, 1 : 1.8 and 1 :2.2. |
|
With sodium carbonate; In water; ethyl acetate; |
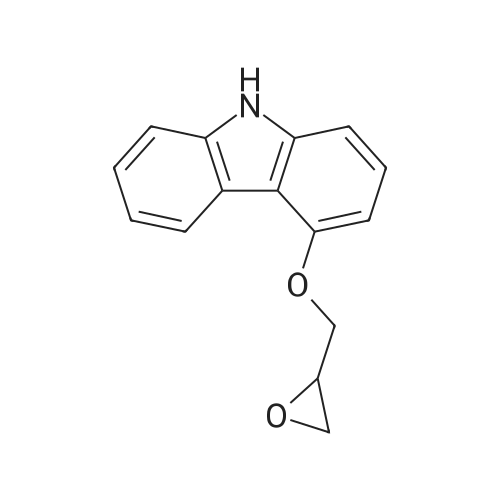
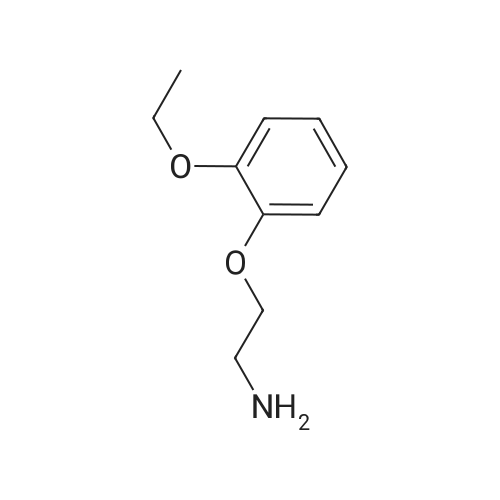
Example 1 Preparation of Carvedilol in Neat Conditions 2-(2-Methoxyphenoxy)ethylamine (III) (4.89 g) was heated to about 100 C., after which <strong>[51997-51-4]4-(oxiran-2-ylmethoxy)-9H-carbazole</strong> (II) (3.31 g) was added portionwise. After approximately 20 minutes, the reaction mixture was cooled to about 70 C., after which water (25 ml) and ethyl acetate (15 ml) were added. The pH of the two-phase mixture was then adjusted to 5 with 2 N hydrochloric acid. The solid that formed, Carvedilol hydrochloride hydrate, is filtered, washed with water (20 ml) followed with ethylacetate (15 ml). The resulting material is reslurried in ethylacetate (50 ml) and water (20 ml) containing 5% Sodium carbonate until the pH reached 7.5. The organic phase was separated and dried over sodium sulfate. The dried solution was concentrated to a turbid solution and cooled overnight to about 4 C. Precipitated carvedilol was isolated by filteration and crystallized from isopropanol. |
|
With sodium carbonate; In water; ethyl acetate; |
Example 2 Preparation of Carvedilol in Neat Conditions 2-(2-Methoxyphenoxy)ethylamine (III) (4.89 g) was heated to about 100 C., after which <strong>[51997-51-4]4-(oxiran-2-ylmethoxy)-9H-carbazole</strong> (II) (3.31 g) was added portionwise. After approximately 20 minutes, the reaction mixture was cooled to about 70 C., after which water (25 ml) and ethyl acetate (15 ml) were added. The pH of the two-phase mixture was then adjusted to 5 with 2 N hydrochloric acid. The solid that formed, Carvedilol hydrochloride hydrate, is filtered, washed with water (20 ml) followed with ethylacetate (15 ml). The resulting material is reslurried in ethylacetate (50 ml) and water (20 ml) containing 5% Sodium carbonate until the pH reached 7.5. The organic phase was separated and dried over sodium sulfate. The dried solution was concentrated to a turbid solution and cooled overnight to about 4 C. Precipitated carvedilol was isolated by filteration and crystallized from methanol. |
|
In dimethyl sulfoxide; at 68 - 72℃; for 18 - 20h;Product distribution / selectivity; |
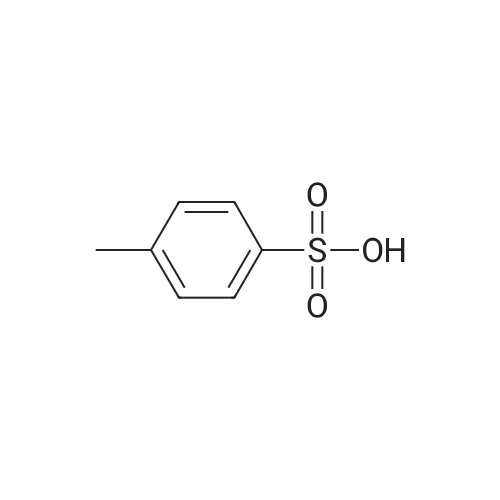
EXAMPLE 1 Preparation of PTSA Salt of Carvedilol a) In a dry reaction flask, 120 gm (0.502 moles) of <strong>[51997-51-4]4-(2,3-epoxypropoxy)carbazole</strong> of Formula II, 188.7 gm (1.13 mole) of 2-(2-methoxy phenoxy)ethylamine, and 1200 ml dimethylsulphoxide (DMSO) were charged under dry nitrogen atmosphere. The reaction mass was heated to about 70 C. till completion of reaction (about 20 hours), and then the reaction mass was cooled to 30 C. and 1200 ml water was added to it. The crude product was extracted with dichloromethane (1200 ml), and the dichloromethane layer was washed with water. The dichloromethane layer was mixed with 240 ml water, followed by the addition of 55.8 gm p-toluene sulphonic acid (PTSA) to attain a pH in the range of 7 to 8. After stirring, the layers were separated and the organic layer was washed with water.; EXAMPLE 4 Preparation of Carvedilol; In a dry reaction flask charged 25.0 g 4-(2,3-epoxy propoxy)carbazole (0.104 moles), 39.35 g of 2-(2-methoxy phenoxy)ethylamine (0.235 moles) in 250 ml dimethyl sulfoxide. The temperature of the reaction mass was raised to about 70 C. under stirring and maintaining the reaction mixture at 68-72 C. for 18-20 hrs. The reaction mass was cooled to about 30 C. and quenched the reaction mass in 250 ml water, stirred and extracted the resultant solution in 250 ml dichloromethane. The organic layer was separated and washed with aqueous sulphuric acid till pH of the washings about 7.0-8.0. The organic layer was separated and further adjusted the pH with the aqueous sulfuric acid to 4.0-4.5 to precipitate carvedilol sulphate salt. The precipitated salt was filtered and taken in 300 ml ethyl acetate and made alkaline with 10% sodium carbonate solution. The reaction mass was stirred and separated the organic layer. The ethyl acetate was distilled under vacuum, and 330 ml toluene was added to it. The solid obtained was filtered and crystallized from ethyl acetate to obtain pure carvedilol (58-62% yield). |
|
In ethyl acetate; for 24h;Reflux; |
In a dry reaction flask, 4-(2,3-epoxy propoxy) carbazole (50 gm.0.21 moles), 2-(2-methoxy phenoxy) ethyl amine (75.5 gm. 0.45 moles) and 500 ml of ethyl acetate were charged and heated to reflux for about 24 hours. After completion of the reaction, solvent was distilled off from the reaction mixture to obtain 125 gm of the residue. Ethyl acetate (312 ml) and water (312 ml) were added to the residue and stirred for about 15 minutes. Reaction mixture pH was adjusted to about 3 with 40 ml (0.68 moles) of phosphoric acid at room temperature. Reaction mixture was stirred for about 11 hours and filtered the solid to obtain carvedilol phosphate. The wet solid thus obtained was slurred in 325 ml of acetone at 26 0C for about 30 minutes. The solid was filtered and dried to obtain the title compound. Yield: 50 gm |
|
lithium bromide; In 1,2-dimethoxyethane; at 50℃; for 24h; |
General Procedure for the Preparation of 1b-1d (FIG. 12, Scheme 4): To a solution of epoxide 6 (1.00 mmol) in anhydrous DME (6 mL) were added amines 7b-7d (2.00 mmol), respectively, and LiBr (catalytic). Each reaction mixture was stirred for 24 h at 50 C. Then the solvent was removed under vacuum, the residues were taken up in ether (10 mL) and washed with water. The aqueous layers were extracted with ether (2×10 mL). The organic layers were washed with brine (25 mL), dried over Na2SO4, evaporated under reduced pressure and the residues were purified by flash chromatography over silica gel to obtain pure 1b-1d. |
| 176 mg |
In ethanol; for 24h;Reflux; |
Compound 5a (100 mg, 0.42 mmol), and 2-(2-methoxyphenoxy)ethanamine (84 mg, 0.50 mmol) were added to ethanol and theresulting heterogeneous solution was reuxed for 24 h. Themixture was cooled to room temperature and ltered through a padof celite and the ltrate was concentrated under reduced pressure.The residue was puried by ash chromatography on silica-gelwith 10% methanol in ethyl acetate. Yielding 83% compound 9a(176 mg) as a white solid. Compound 9b was synthesized followingthe procedure of preparation 9a. |
|
In ethyl acetate; for 24h;Reflux; |
Example 5: Preparation of Carve dilol using 2-(2-methoxyphenoxy) ethylamine solid: In a dry reaction flask, 4-(2,3-epoxy propoxy) carbazole (50 gm), 27(2- methoxy phenoxy) ethyl amine solid (75.5 gm) and 500 ml of ethyl acetate were charged and heated to reflux for about 24 hours. After completion of the reaction, solvent was distilled off from the reaction mixture to obtain 117 gm of the residue. Ethyl acetate ( 175 ml) was added to the residue and stirred for about 12 hours at room temperature. The suspension was cooled to O0C, filtered and dried to get 65 gm of the title compound. M.R: 115 C- 1 17C Purity by HPLC: 99.74% |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +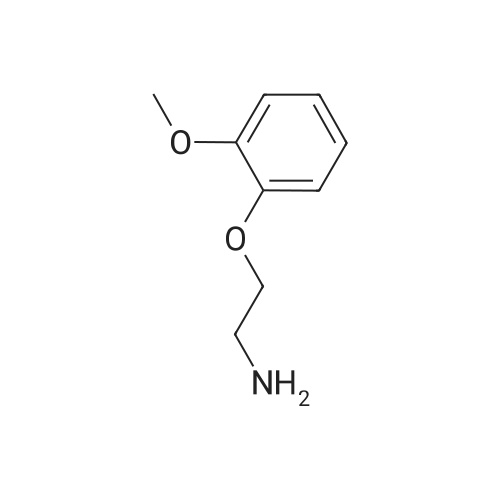

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping