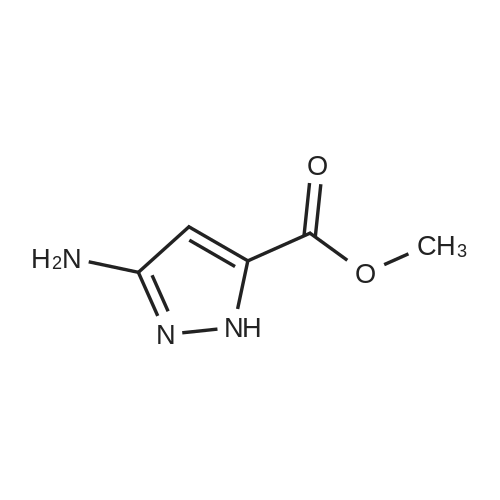
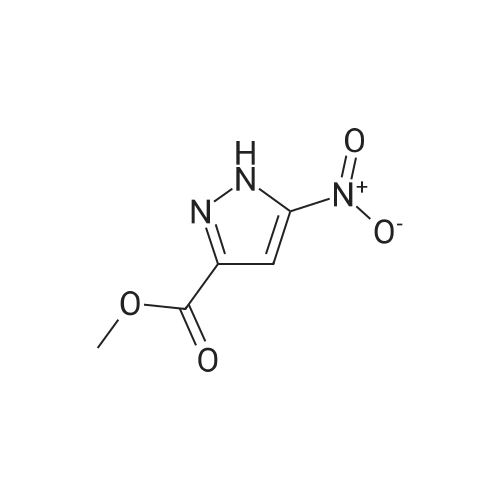
| 95% |
With thionyl chloride; at 80℃; for 16h; |
To a stirred solution of 3-nitro-lH-pyrazole-5-carboxylic acid(A58, 25.0 g, 159.14 mmol) in Methanol(250 mL) was added dropwise SOCk(23.09 mL, 318.28 mmol) mL). The reaction mixture was stirred for 16 h at 80C. After completion, addition of n-hexane(500 mL) solid precipitated, filtered and dried under reduced pressure to afford methyl 3-nitro-lH-pyrazole-5-carboxylate A59 as a white solid. Yield: 26.0 g(95%) LC-MS(ES) m/z : 170.14[M+H]+. |
| 93% |
With sulfuric acid; for 5h;Reflux; |
Commercial 3-nitro-1H-pyrazole-5-carboxylic acid (99.6%purity, Aldrich) (1.54 g, 10mM)was dissolved in methanol (15 mL), and 95%H2SO4 (0.14 mL) was added.The mixture was refluxed for 5 h. The solvent was evaporated under reduced pressure. A precipitatewas dissolved in ethyl acetate and washed with 5% NaHCO3 (2 x10 mL) and water (1 x10 mL).The organic layer was dried with MgSO4, and the ethyl acetate was evaporated. The residue wasdissolved in dichloromethane and purified on a silica-gel column (60H Merck 1.05553) eluted withDCM:MeOH, from 2%±6% of MeOH; crystallization from a mixture of diethyl ether/hexane. Yield:1.28 g, 93%. 1H NMR (400MHz, DMSO-d6) delta(ppm): 15.25 (1H, s, N-HPz), 7.54 (1H, s, C-HPz), 3.91 (3H,s, CH3) (Figure S19). Melting point by DSC: 148 C (Figure S24). TLC CHCl3/MeOH/AcOH (90:8:2)Rf = 0.74. |
| 89.3% |
With thionyl chloride; at 0℃; for 2h;Reflux; |
Step 11: Preparation of 5-Nitro-2H-pyrazole-3-carboxylic acid methyl ester A lOmL single-neck round-bottomed flask was charged with 5-nitro-lH-pyrazole-3-carboxylic acid (4.0 g, 25.5 mmol) and anhydrous MeOH (40.0 ml). The reaction mixture was cooled to 0 C in an ice/water cooling bath. To this mixture, thionyl chloride (7.88 g, 4.83 ml, 66.2 mmol) was added dropwise. After the addition was complete, the bath was removed and the reaction mixture was heated at reflux for 2 h. The reaction mixture was then concentrated to dryness under reduced pressure to give the desired product (4.36 g, 89.3%). (M+Na)+ = 198.9 m/e |
| 81% |
|
To a solution of 3-nitro-1H-pyrazole-5-carboxylic acid (2.00 g, 12.73 mmol) in methanol (80 mL) was added thionyl chloride (1.00 ml_, 13.70 mmol). The reaction mixture was heated to reflux for 18 hours. The solvent was removed in vacuo, and the residue was dissolved in ethyl acetate and washed with saturated sodium bicarbonate, water and brine. The organic phase was dried over anhydrous sodium sulfate and filtered. The filtrate was concentrated in vacuo to afford methyl 3-nitro-1H- pyrazole-5-carboxylate in 81 % yield (1.77 g): 1H NMR (300 MHz, CDCI3) delta 9.97 (br s, 1 H), 7.39 (s, 1H), 3.99 (s, 3H); MS (ES+) m/z 171.0 (M). |
| 81% |
With thionyl chloride; at 0℃; for 2.33333h;Reflux; |
To a sobr& of 3-nibD-lH-pa7de-5-ca1boxy& acid (69.75 g, 444 intol) (ArkPhant in MDH (1L)was ad&dthirckhide (S4 nL, llS4numl) O C. Th nuetirewas sth1 £Srabciat2J ittat about 0 C then Inted to D?fbIx fc about 2k. Th iemlbrg sobxon was oDirentated urderpressuie to give fl1 (61.25 g, Si %): LCIMS (Table1, Metlvd I) R = 1.42 ntha.; MS m&: 169 (M-i-H). |
| 74% |
With thionyl chloride; at 0 - 70℃; for 4h; |
To a stirred solution of 3-nitro-1H-pyrazole-5-carboxylic acid 19 (2.0 g,11.69 mmol)) in methanol (50 mL), thionyl chloride (2.07 g, 17.54 mmol) was charged at0C, in portions. The reaction mixture was heated at 70C for 4 hour. The reaction p1ixture was evaporated and basified with saturated sodium bicarbonate solution to pH 9. The aqueous layer was extracted with dichloromethane (2 x 100 mL). The combined organic extract was washed with water (3 x 30 mL). The organic layer was dried (Na2SO4), filtered and evaporated to obtain methyl 3-nitro-1H-pyrazole-5-carboxylate 20 (1.5 g, 74% yield) as brown sticky solid. MS: 171.03 (M+H). |
| 70% |
With sulfuric acid; at 20 - 65℃; for 89h; |
Step 1: Methyl 3-nitro-lH-pyrazole-5-carboxylate [00198] A stirred solution of 3-nitro-lH-pyrazole-5-carboxylic acid (940 mg, 5.98 mmol) in MeOH (10 mL) at 20 C was treated cautiously with cone. H2S04 (1 mL). The reaction mixture was heated to 65 C for 89 h, then concentrated to dryness. The resulting white suspension was triturated with water (10 mL) and the resulting precipitate collected by filtration to yield the title compound as a white solid (718 mg, 70%). LCMS (ES+) 172 (M+H)+. |
| 69% |
With thionyl chloride; at 0℃; for 16h;Reflux; |
5.1.45 Methyl 3-nitro-1H-pyrazole-5-carboxylate (9) To a solution of 8 (20.0 g, 127.32 mmol) in anhydrous methanol (70 mL) was added SOCl2 (10.2 mL, 140.11 mmol) dropwise at 0 C. The resulting mixture was heated at reflux for 16 h, and then concentrated in vacuo. The residue was dissolved in ethyl acetate (200 mL) and washed with saturated NaHCO3 solution (40 mL * 2), water (40 mL), and brine (40 mL), dried over anhydrous Na2SO4, and filtered. The filtrate was concentrated in vacuo, and the residue was crystallized from ethyl acetate/hexane to afford 9 as an off-white solid (15.0 g, 69%). 1H NMR (300 MHz, CDCl3) delta 11.52 (br s, 1H), 7.41 (s, 1H), 4.01 (s, 3H); MS (ES-) m/z 170.0 (M-1). |
| 13.8% |
|
Step I - Preparation of5-nitro-2H-pyrazole-3-carboxylic acid methyl ester (525):; [0136] To 5-nitro-2H-pyrazole-3-carboxylic acid (524, 10.0 g, 0.0637 mol) in methanol (100.0 inL) was added concentrated sulfuric acid (1.00 mL, 0.0180 mol). The reaction was stirred at room temperature overnight. The reaction was poured into aqueous potassium carbonate and extracted with ethyl acetate. The organic layer was dried over anhydrous sodium sulfate and filtered. The filtrate was concentrated and purified by silica gel column chromatography eluting with 20% ethyl acetate in hexane to give a white solid (525, 1.5 g, 13.8%). |
|
With sulfuric acid; for 20h;Heating / reflux; |
delta-Nitro^H-pyrazole-S-carboxylic acid methyl ester: A solution of delta-Nitro^H-pyrazole-S-carboxylic acid (5 gm, 32 mmol) in100 ml of 2% sulfuric acid methanol was refluxed for 20 hours. The mixture was cooled to room temperature and added to saturated sodium bicarbonate. The mixture was extracted with ethyl acetate three times to obtain 3.59 gm of title product after drying over magnesium sulfate, filtering and evaporating to dryness. ESI M+1 =171. |
|
With sulfuric acid; at 65℃; |
To a solution of 3-nitro-JH-pyrazole-5-carboxylic acid (1 57 g, 10.0 mmol) in MeOH(20 mL) was added conc.fl2S04 (2.0 mL). The resulting mixture was stirred at 65 C overnight.Then the mixture was concentrated in vacuo to gtve a residue, which was purified by silica gelcolumn (DCM/J1eOH '" 50/l) to afford methyl 3-nitro-1 H-pyrazole-5-carboxy late (1.42 g, yield:83?--o) as yellow solid. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping