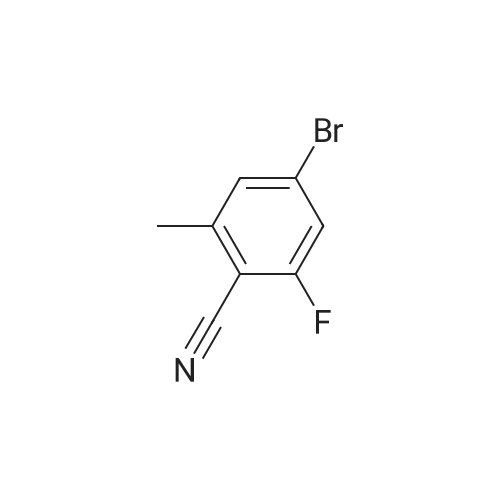
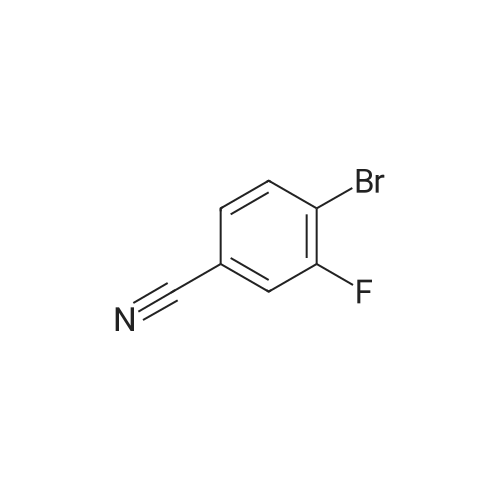
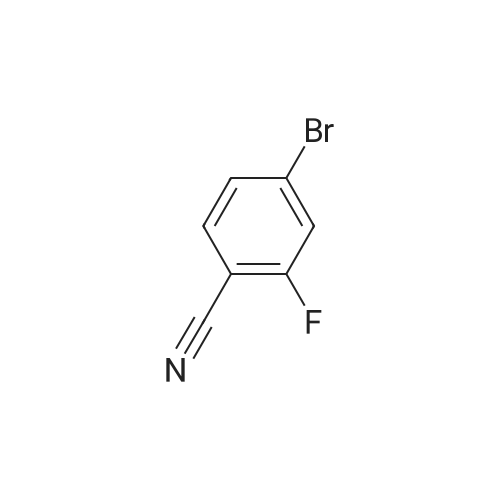
| 33% |
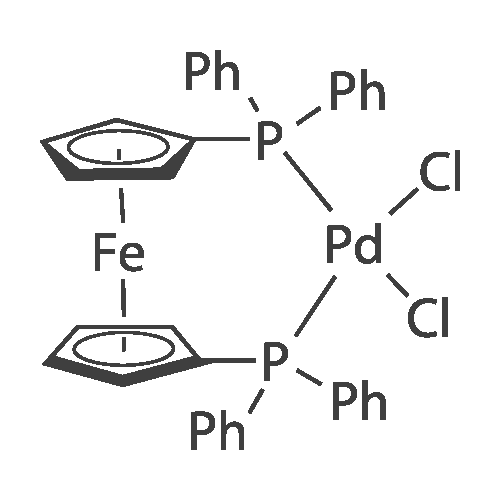
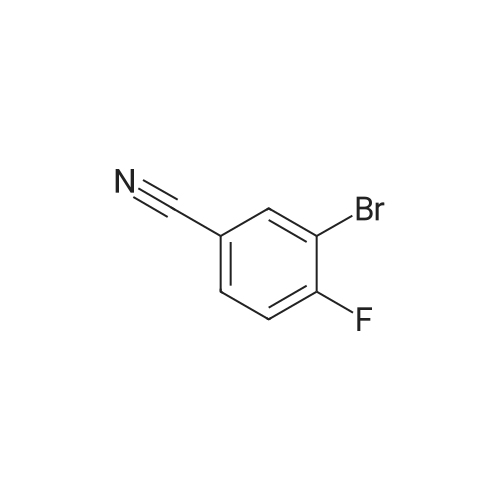
With potassium acetate; sodium carbonate; In water; ethyl acetate; N,N-dimethyl-formamide; |
A mixture of 6-bromo-8-methoxy-4,4-dimethyl-1,4-dihydro-2H-3,1-benzoxazin-2-one (1.6 g, 5.6 mmol), bis(pinacolato)diboron (1.6 g, 6.3 mmol), potassium acetate (1.5 g, 15.3 mmol), and [1,1'-bis(diphenylphosphino)ferrocene]palladium (II) chloride (1:1 complex with methylene chloride, 0.5 g, 0.6 mmol) in DMF (30 mL) was subject to a positive flow of nitrogen to remove oxygen and then heated at 85° C. under a blanket of nitrogen for 18 hours. The reaction mixture was allowed to cool to ambient temperature, treated with 3-bromo-5-fluoro-benzonitrile (1.2 g, 6 mmol), [1,1'-bis(diphenylphosphino)ferrocene]palladium (II) chloride (1:1 complex with methylene chloride, 0.5 g, 0.6 mmol), and sodium carbonate (2 g, 19 mmol) in water (10 mL). The resulted solution was heated at 85° C. for 3 hours under a blanket of nitrogen, cooled to rt, and treated with brine (50 mL). Ethyl acetate (100 mL) was added, organic layer was separated, dried (MgSO4), and evaporated. The residue was purified by a flash silica gel column chromatography (THF:hexane/2:3) to yield 3-(4,4-dimethyl-8-methoxy-2-oxo-1,4-dihydro-2H-3,1-benzoxazin-6-yl)-5-fluorobenzonitrile as a white solid (0.6 g, 33percent): mp 252-253° C.; 1H-NMR (DMSO-d6) delta9.76 (s, 1H), 8.21 (s, 1H), 8.07 (d, 1H, J=10.6 Hz), 7.82 (m,1H), 7.39 (s 1H), 7.36 (s, 1H), 3.93 (s, 3H), 1.66 (s, 6H). MS (ESI) m/z 325 [M-H]-. |
| 33% |
With potassium acetate; sodium carbonate; In water; ethyl acetate; N,N-dimethyl-formamide; |
A mixture of 6-bromo-8-methoxy-4,4-dimethyl-1,4-dihydro-2H-3,1-benzoxazin-2-one (1.6 g, 5.6 mmol), bis(pinacolato)diboron (1.6 g, 6.3 mmol), potassium acetate (1.5 g, 15.3 mmol), and [1,1'-bis(diphenylphosphino)ferrocene]palladium (II) chloride (1:1 complex with methylene chloride, 0.5 g, 0.6 mmol) in DMF (30 mL) was subject to a positive flow of nitrogen to remove oxygen and then heated at 85° C. under a blanket of nitrogen for 18 hours. The reaction mixture was allowed to cool to ambient temperature, treated with 3-bromo-5-fluoro-benzonitrile (1.2 g, 6 mmol), [1,1'-bis(diphenylphosphino)-ferrocene]palladium (II) chloride (1:1 complex with methylene chloride, 0.5 g, 0.6 mmol), and sodium carbonate (2 g, 19 mmol) in water (10 mL). The resulted solution was heated at 85° C. for 3 hours under a blanket of nitrogen, cooled to rt, and treated with brine (50 mL). Ethyl acetate (100 mL) was added, organic layer was separated, dried (MgSO4), and evaporated. The residue was purified by a flash silica gel column chromatography (THF:hexane/2:3) to yield 3-(4,4-dimethyl-8-methoxy-2-oxo-1,4-dihydro-2H-3,1-benzoxazin-6-yl)-5-fluorobenzonitrile as a white solid (0.6 g, 33percent): mp 252-253° C.; 1H-NMR (DMSO-d6) delta9.76 (s, 1H), 8.21 (s, 1H), 8.07 (d, 1H, J=10.6 Hz), 7.82 (m, 1H), 7.39 (s 1H), 7.36 (s, 1H), 3.93 (s, 3H), 1.66 (s, 6H). MS (ESI) m/z 325 [M-H]-. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping