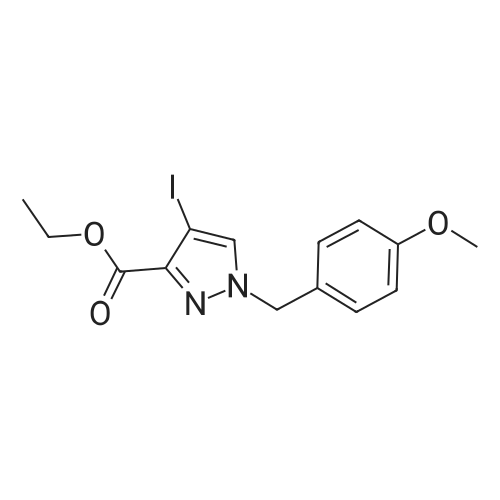
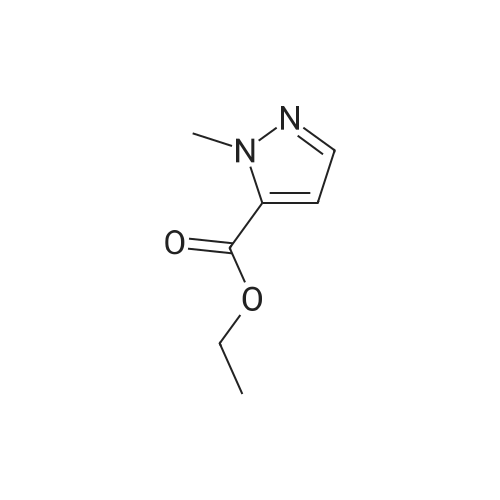
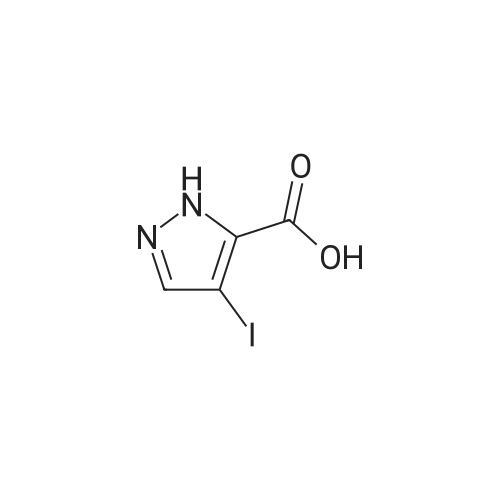
| 69.32% |
With ceric ammonium nitrate; iodine; In acetonitrile; at 20℃; for 16h; |
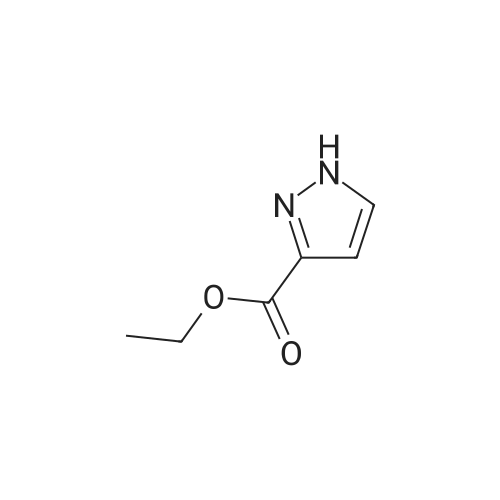
Iodine (54.33 g, 214.07 mmol) was added into a solution of <strong>[5932-27-4]ethyl 1H-pyrazole-3-carboxylate</strong> (30 g, 214.07 mmol) in acetonitrile, followed by the addition of ammonium cerium nitrate (117.36 g, 214.07 mmol). The mixture was stirred at 20°C for 16 h, added with a cold 5percent aqueous NaHSO3 solution (400 mL), filtered through diatomaceous earth, and washed with water (200 mL) and ethyl acetate (500 mL). The filtrate was evaporated in a rotary evaporator to remove the organic solvent therein, and then extracted with ethyl acetate (200 mL * 5). The resulting organic phase was washed with water (50 mL * 2) and saturated brine (500 mL), dried over anhydrous sodium sulfate, filtered and evaporated. The residue obtained was purified by column chromatography to give the title compound as a brown liquid (40 g, 69.32percent). 1H NMR (400 MHz, DMSO-d6) delta=7.99 (br. s., 1H), 4.29 (q, J=7.1 Hz, 2H), 1.31 (t, J=7.0 Hz, 3H). |
| 52% |
With ammonium cerium (IV) nitrate; iodine; In acetonitrile; at 20℃; for 20h; |
Example 15, Step B[00153] To a solution of compound 15b (38.5 g, 275 mmol) in CH3CN (700 mL) was added iodine (69.8 g, 275 mmol) followed by eerie ammonium nitrate (150.7 g, 275 mmol). The reaction mixture was then stirred for 12 hours at r.t. Additional iodine (17.4 g) was added and stirring continued for 8 h, following which a cold solution of 5percent NaHS03was added to the reaction mixture. The white precipitate was filtered through a celite pad and washed with water and EtOAc. The filtrate layers were separated, the aqueous phase extracted with EtOAc and the organic phases were washed with water, dried over MgS04, filtered and solvent evaporated in vacuo to afford compound 15c (38 g, 52percent) as a slightly yellow solid. |
| 52% |
|
Example 15, Step B[00153] To a solution of compound 15b (38.5 g, 275 mmol) in CH3CN (700 mL) was added iodine (69.8 g, 275 mmol) followed by eerie ammonium nitrate (150.7 g, 275 mmol). The reaction mixture was then stirred for 12 hours at r.t. Additional iodine (17.4 g) was added and stirring continued for 8 h, following which a cold solution of 5percent NaHSOawas added to the reaction mixture. The white precipitate was filtered through a celite pad and washed with water and EtOAc. The filtrate layers were separated, the aqueous phase extracted with EtOAc and the organic phases were washed with water, dried over MgS04, filtered and solvent evaporated in vacuo to afford compound 15c (38 g, 52percent) as a slightly yellow solid. |
| 39% |
With ammonium cerium (IV) nitrate; iodine; In acetonitrile; at 20℃; |
b. 4-Iodo-<strong>[5932-27-4]1H-<strong>[5932-27-4]pyrazole-3-carboxylic acid ethyl ester</strong></strong> (Intermediate 74b) A suspension of Intermediate 74a (5.00 g, 35.7 mmol) in acetonitrile (90 mL) was treated with iodine (9.10 g, 35.7 mmol) then ceric ammonium nitrate (19.6 g, 35.7 mmol) and the mixture was stirred at RT overnight. Another portion of iodine (2.28 g, 9.0 mmol) was added and the mixture was stirred for a further 24 h then treated with ice-cold aqueous sodium hydrogensulphite solution (5percent, 100 mL). The mixture was filtered through Celite rinsing with EtOAc and water. The phases were separated and the aqueous phase was extracted with EtOAc (2*). The combined organic layers were washed with water and brine, dried (Na2SO4), filtered and evaporated in vacuo. The resulting solid was triturated with ether/cyclohexane, filtered off, washed with cyclohexane and dried at 50° C. in vacuo to give the title compound (3.70 g, 39percent). LCMS (Method 3): Rt 2.88 min, m/z 267 [MH+] (weak). |
| 39% |
With ammonium cerium (IV) nitrate; iodine; In acetonitrile; at 20℃; |
A suspension of Intermediate 74a (5.00 g, 35.7 mmol) in acetonitrile (90 mL) was treated with iodine (9.10 g, 35.7 mmol) then eerie ammonium nitrate (19.6 g, 35.7 mmol) and the mixture was stirred at RT overnight. Another portion of iodine (2.28 g, 9.0 mmol) was added and the mixture was stirred for a further 24h then treated with ice-cold aqueous sodium hydrogensulphite solution (5percent, 100 mL). The mixture was filtered through Celite rinsing with EtOAc and water. The phases were separated and the aqueous phase was extracted with EtOAc (2x). The combined organic layers were washed with water and brine, dried (Na2S04), filtered and evaporated in vacuo. The resulting solid was triturated with ether/cyclohexane, filtered off, washed with cyclohexane and dried at 50°C in vacuo to give the title compound (3.70 g, 39percent). LCMS (Method 3): Rt 2.88 min, m/z 267 [MH+] (weak). |
| 1 g |
With N-iodo-succinimide; In N,N-dimethyl-formamide; at 20 - 60℃; for 1.5h; |
To a solution of ethyl 3-pyrazolecarboxylate (1.0 g) in N,N-dimethylformamide (7.0 mL) was added N-iodosuccinimide (1.6 g), and the mixture was stirred at room temperature for 1 hour, warmed to 60°C, and then stirred for 0.5 hours. The reaction solution was returned to room temperature, saturated aqueous sodium bicarbonate and sodium sulfite were added thereto, and the mixture was extracted with ethyl acetate. The organic layer was washed with brine and then dried over sodium sulfate. The solvent was removed under reduced pressure and the obtained residue was purified by silica gel column chromatography (hexane-ethyl acetate) to obtain a compound 0215-1 (1.0 g) as a pale yellow solid. |
|
With ammonium cerium (IV) nitrate; iodine; In acetonitrile; at 20℃; |
To a solution of <strong>[5932-27-4]ethyl 1H-pyrazole-3-carboxylate</strong> (2 g, 14.3 mol, 1.0 eq) and I2 (3.6 g, 14.3 mmol, 1.0 eq) in ACN (14 mL) was added CAN (1.6 g, 2.86 mmol, 0.2 eq) at RT. The reaction mixture was stirred at RT overnight, and was monitored by reverse-phase analytical HPLC. When starting material was consumed, the reaction mixture was concentrated in vacuo to afford a crude solid which was slowly poured into a saturated Na2S2O3 solution and H2O (1:1) with stirring. The light-yellow suspension was filtered and the filter cake was washed with H2O. The resulting near colorless solid was dried under vacuum and used without further purification.1H NMR (400 MHz, CDCl3) delta 7.86 (s, 1H), 4.46 (q, J=7.2Hz, 2H), 1.45 (t, J=7.2 Hz, 3H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping