| 95% |
|
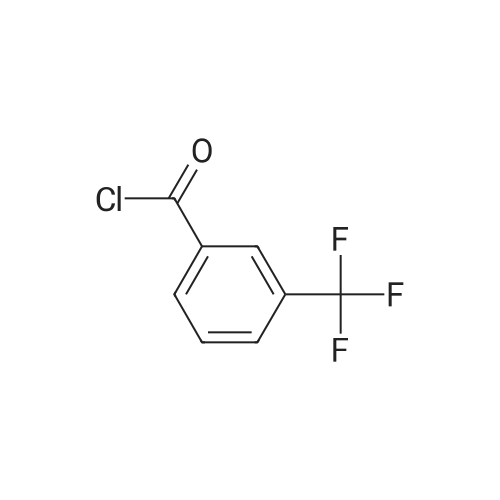
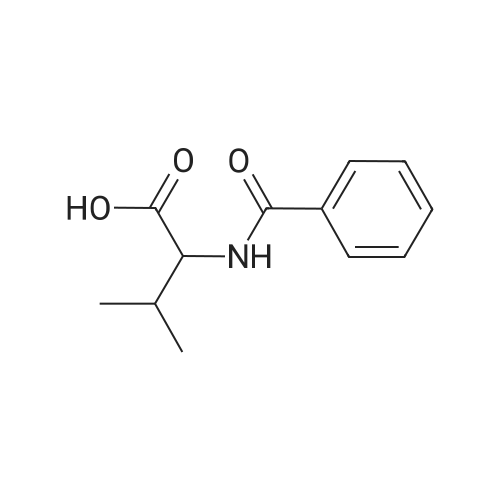
A 12-L 4-neck round bottom flask equipped with a thermocouple controller, mechanical stirrer, heating mantle, condenser and a nitrogen in/outlet adapter was charged with gycine (1, Alfa Aesar) (318 g; 4.19 mol), acetonitrile (1.2 L), and a solution of sodium hydroxide (5.31 L; 10.62 mo) and the mixture was cooled to 4 C. with stirring. A solution of 3-(trifluoromethyl)benzoyl chloride (2, Alfa Aesar) (885.0 g; 4.12 mol) (640 mL) in acetonitrile (0.75 L) (total 1.39 L) was added dropwise over 2 h while the internal temperature was maintained between 4-6 C., and the slightly orange-pinkish solution was stirred at 4 C. for an additional 30 min. The reaction was acidified to pH=3 with conc. 37% HCl solution (400 mL added over 30 min) at 0-6 C., and stirred for 1 h at 0 C. (until a slightly yellowish suspension resulted). The solid was collected by filtration, washed with cold (0 C.) deionized ("D.I") H2O (300 mL*2), dried under air-suction for 2 h, and then placed in a drying oven at 60 C. under house vacuum (120 mmHg) for 20 h to afford pure 3 as an off-white solid. The filtrate was extracted with EtOAc (1 L*2), and the combined organic phases washed with brine (300 mL), and concentrated at 66 C. under house vacuum and then high vacuum (20 mmHg) to give crude product as an off-white waxy solid, which was triturated and sonicated with toluene (1 L) and stirred at 10 C. for 1 h. The resulting solid was collected by filtration, washed with hexanes (50 mL*2), dried in an vacuum oven at 50 C. under house vacuum to afford additional pure title compound, 3, as an off-white solid. The structure of 3 was confirmed with its 1H-NMR. |
| 91% |
With hydrogenchloride; sodium hydroxide; In acetonitrile; at 0 - 3℃; for 1h;pH 2 - 3; |
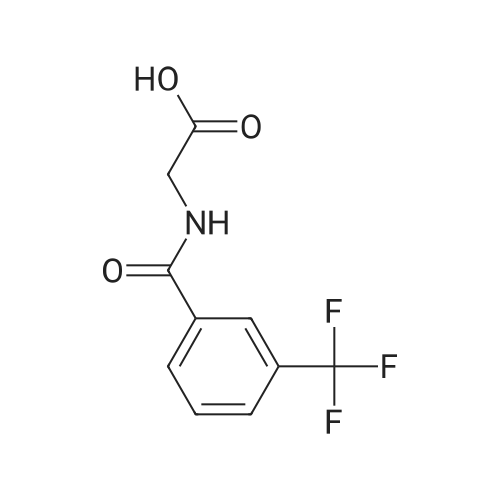
Manufacturing example 1: (3-trifluoromethylbenzoylamino)-acetic acid [Show Image] Glycine 0.763 g (10.16 mmol) were suspended in acetonitrile 20 ml and 2 M NaOH aqueous solution 12.7 ml (25.40 mmol, 2.5 eq.) were also added. After chilling at 0-3C, 2.12 g (10.16 mmol, 1.0 eq.) of 3- (trifluoromethyl) -benzoyl chloride were diluted with 4 ml acetonitrile and added dropwise slowly to reaction mixture. After one hour agitation at the same temperature, pH was controlled to 2 to 3 with 3N hydrochloric acid aqueous solution. After keeping upright at room temperature, upper organic solution was separated, and lower aqueous solution was extracted with ethylacetate three times. Those organic solutions obtained as described above were brought all together, dried with anhydrous magnesium sulfate and concentrated, removing the solvent under decompression. Residues were solidified with toluene, filtered, washed with normal hexane and 2.28 g (91%) target compound as white solid were yielded. 1H NMR(400MHz,DMSO-d6) 3.94(2H,d), 7.74(1H,t), 7.93(1H,d), 8.16(1H,d), 8.20(1H,s), 9.12(1H,t) |
| 91% |
With hydrogenchloride; sodium hydroxide; In water; acetonitrile; at 0 - 3℃; for 1h;pH 2 - 3; |
Manufacturing Example 1 (3-trifluoromethylbenzoylamino)-acetic acid Glycine 0.763 g (10.16 mmol) was suspended into acetonitrile 20 ml and 2M NaOH aqueous solution 12.7 ml (25.40 mmol, 2.5 eq.) was also added. After chilling at 0-3 C., 2.12 g (10.16 mmol, 1.0 eq.) of 3-(trifluoromethyl)-benzoyl chloride was diluted with 4 ml acetonitrile and was added dropwise slowly to reaction mixture. After one hour agitation at same temperature, pH was controlled to 2 to 3 with 3N hydrochloric acid aqueous solution. After keeping upright at room temperature, upper organic solution was separated, and lower aqueous solution was extracted with ethylacetate three times. Those organic solution obtained as above was brought all together, dried with anhydrous magnesium sulfate and concentrated removing its solvent under decompression. Residues was solidified with tolene, filtered, washed with normal hexane and 2.28 g (91%) target compound as white solid was yielded. 1H NMR (400 MHz, DMSO-d6) 3.94 (2H, d), 7.74 (1H, t), 7.93 (1H, d), 8.16 (1H, d), 8.20 (1H, s), 9.12 (1H, t) |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping