|
With hydrogen bromide; bromine; acetic acid; In chloroform; for 0.5h; |
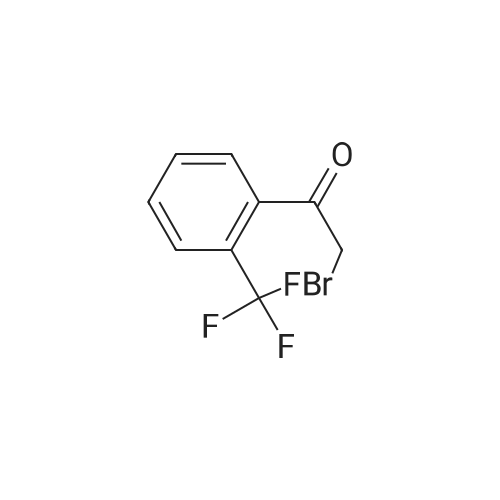
To a solution of l-(2-(trifluoromethyl)phenyl)ethanone (71.0 g, 377.0 mmol)and HBr (2.0 mL, 45% solution of AcOH) in chloroform (500.0 mL) was added the solution of dibromide (60.3 g, 377.0 mmol) in chloroform (200.0 mL). After addition, the solution was stirred for 30 min, then the solvent was evaporated and the residue was used in the next step directly. MS (ESI) calcd for C9H6BrF30: 265.96. |
|
With bromine; In diethyl ether; chloroform; at 10 - 35℃; for 1h; |
Reference Example 25 Ethyl 2-cyano-4-oxo-4-[(2-trifluoromethylphenyl)butanoate 2'-(Trifluoromethyl)acetophenone (10.0 g) was dissolved in chloroform (30 mL) and diethyl ether (30 mL), a solution of bromine (8.50 g) in chloroform (20 mL) was added dropwise while maintaining the reaction temperature at not higher than 25C. After the dropwise addition, the mixture was stirred at room temperature for 1 hr, water was added to the reaction mixture and the mixture was extracted with chloroform. The extract was washed with saturated brine, dried over anhydrous magnesium sulfate, concentrated under reduced pressure to give crude 1-bromo-1-(2-trifluoromethylphenyl)ethanone. |
|
With bromine; In diethyl ether; at 20℃; for 0.5h; |
General procedure: To a stirred solution of 2-methylacetophenone 8b (13.42 g, 100 mmol) in Et2O (100 mL) was added dropwise bromine (16.0 g, 100 mmol), and the mixture was stirred at room temperature for 30 min, poured into ice H2O, extracted with Et2O. The extract was washed with brine, dried over anhydrous MgSO4, filtered and concentrated under reduced pressure to obtain an crude oil of 2-bromo-1-(2-methylphenyl)ethanone. |
|
With N-Bromosuccinimide; toluene-4-sulfonic acid; In water; acetonitrile; at 80℃; for 4h;Inert atmosphere; |
A 200 mL flask was charged with 2′-trifluoromethylacetophenone (9.41 g, 50 mmol), N-bromosuccinimide (9.79 g, 55 mmol, 1.1 eq.), p-toluenesulfonic acid (10.46 g, 55 mmol, 1.1 eq.) and MeCN (100 mL) under nitrogen stream and the mixture was stirred for 4 hours at 80 C. The mixture was allowed to cool to room temperature and then MeCN was removed by an evaporator. CHCl3 (150 mL) and H2O (50 mL) were added to the residue and the lower layer was separated and then washed with saturated aqueous NaHCO3 (50 mL) twice and brine (50 mL). The solution was dried over anhydrous MgSO4 and filtered and then solvents were removed in an evaporator to obtain the crude product as yellow oil (13.66 g) in >99.9% yield. This product was identified as 2-Bromo-2′-trifluoromethylacetophenone contaminated with some dibromo compound. 1H-NMR (CDCl3), delta (ppm): 4.36 (s, 2H), 7.48-7.50 (m, 1H), 7.60-7.64 (m, 2H), 7.72-7.75 (m, 1H), 19F-NMR (CDCl3), delta in ppm, standard: C6F6=-162.2 ppm: -58.68. |
|
With bromine; In dichloromethane; for 1.5h; |
2-Amino-4-(2-trifluoromethyl-phenyl)-thiazole-5-carboxylic acid amide (V.17); Step 1; To a solution of 20.00 g (0.11 mole) of 2'-(trifluoromethyl)acetophenone in 200 mL of dichloromethane was added 5.5 mL (0.11 mole) of bromine over 90 minutes. A stream of nitrogen was bubbled through the reaction mixture for 15 minutes. The mixture solvent was removed under reduced pressure. The residue was dissolved with ethanol, and then concentrated under reduced pressure to give 27.25 g of 2-bromo-1-(2-trifluoromethyl-phenyl)-ethanone as a yellow oil, which was used in the next step without further purification. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping