|
In ethyl acetate; at 20℃; for 40h;Product distribution / selectivity; |
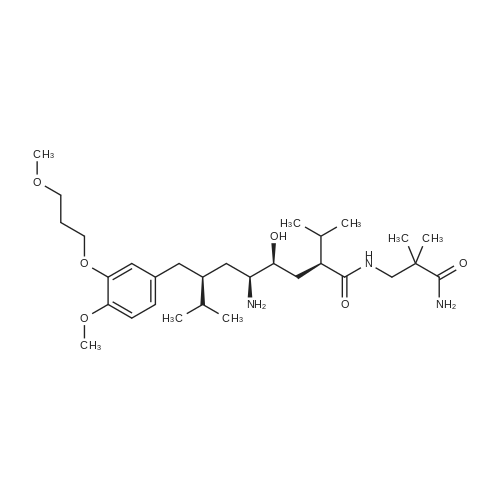
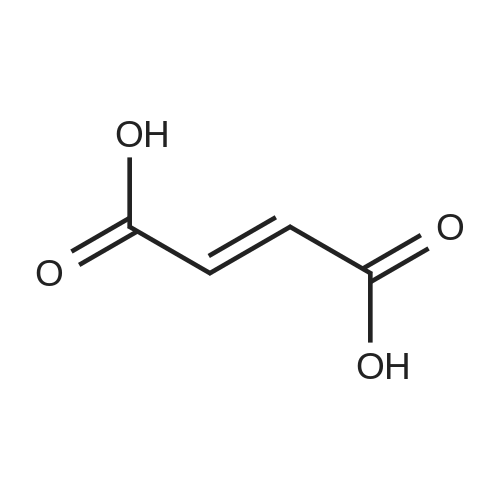
Example 43: <strong>[173334-57-1]Aliskiren</strong> base (50 mg) was dissolved in 1 ml of ethyl acetate and fumaric acid (5.3 mg) was added. The suspension was stirred for 40 hours at room temperature. The solid was then filtered from the suspension and analyzed by powder XRD. (See Figure 9.) |
|
In ethanol; n-heptane; at 20℃;Product distribution / selectivity; |
Example 23: <strong>[173334-57-1]Aliskiren</strong> base (50 mg) was dissolved in 0.5 ml of heptane-ethanol:10 volume-1 volume, and fumaric acid (5.3 mg) was then added. The suspension was stirred at room temperature overnight and became unstirrable. 0.5 ml of heptane : ethanol in a 10:1 volume ratio was added and the suspension was stirred 20 hours at room temperature. A sample of the solid from the suspension was then analyzed by powder XRD. |
|
In acetonitrile; at 20℃; for 40h;Product distribution / selectivity; |
Example 32: <strong>[173334-57-1]Aliskiren</strong> base (50 mg) was dissolved in 1 ml of acetonitrile, and fumaric acid (5.3 mg) was then added. The suspension was stirred for 40 hours at room temperature. The solid was then filtered from the suspension and analyzed by powder XRD. |
|
In ethanol; at 20℃;Product distribution / selectivity; |
Example 67: <strong>[173334-57-1]Aliskiren</strong> base (5.5 g) was dissolved in 100 ml of ethanol, and fumaric acid (0.6 g) was then added. The suspension was stirred at room temperature and became a solution. The ethanol was then evaporated to dryness under vacuum. The resulting solid was analyzed by powder XRD and found to be amorphous form of aliskiren hemifumarate. |
|
In tert-butyl methyl ether; at 20℃;Product distribution / selectivity; |
Example 62: <strong>[173334-57-1]Aliskiren</strong> base (50 mg) was dissolved in 0.5 ml of MTBE, and fumaric acid (5.3 mg) was then added. The suspension was stirred at room temperature overnight and became unstirrable. 0.5 ml of MTBE was added and the suspension was stirred 20 hours at room temperature. A sample of the solid from the suspension was analyzed by powder XRD. |
|
In 2-methylpropyl acetate; at 20℃;Product distribution / selectivity; |
Example 22: <strong>[173334-57-1]Aliskiren</strong> base (50 mg) was dissolved in 0.5 ml of iso-butyl acetate, and funiaric acid (5.3 mg) was added to the solution. The resulting suspension was stirred at room temperature overnight and became unstirrable. 0.5 ml of Iso-butyl acetate was added and the suspension was stirred 20 hours at room temperature. The solid was then filtered from the suspension and analyzed by powder XRD. |
|
In Diethyl carbonate; at 20℃; for 40h;Product distribution / selectivity; |
Example 42: <strong>[173334-57-1]Aliskiren</strong> base (50 mg) was dissolved in 1 ml of diethylcarbonate, and fumaric acid (5.3 mg) was then added. The suspension was stirred for 40 hours at room temperature. The solid was then filtered from the suspension and analyzed by powder XRD. |
|
In ethanol; |
9 g of aliskiren were dissolved in 26 ml of ethanol and mixed with 0.95 g of fumaric acid. The mixture was filtered off and the solvent was evaporated. The residue was dissolved in 220 ml of acetonitrile and stirred at ambient temperature. The resulting solution was seeded with 50 mg of aliskiren hemifumarate and agitated at ambient temperature for 17 hours. The suspension was cooled to -10C and filtered off by suction after 1 hour. The residue was washed with 3 x 25 ml of acetonitrile and then dried in a vacuum oven at 30C for 1 hour. The aliskiren hemifumarate was obtained as white crystals (7.6 g). 1H-NMR (DMSO-d6) δ/ppm: 7.62 (t, 1H), 7.20 (s, 1H), 6.83-6.79 (m, 3H), 6.69 (dd, 1H), 6.35 (s, 1H), 3.97 (t, 2H), 3.71 (s, 3H), 3.46 (t, 2H), 3.28 (m, 1H), 3.23 (s, 3H), 3.18-3.08 (m, 2H), 2.57 (m, 1H), 2.46-2.25 (m, 3H), 1.92 (m, 2H), 1.79 (m, 1H), 1.69-1.52 (m, 3H), 1.41-1.21 (m, 3H), 1.03 (s, 6H), 0.85-0.76 (m, 12H). 13C-NMR (DMSO-δ6) d/ppm: 178.5, 174.7, 170.2, 147.8, 147.2, 135.9, 133.4, 121.1, 114.2, 112.0, 69.3, 68.7, 65.4, 57.9, 55.5, 54.2, 48.9, 46.3, 42.6, 40.2, 36.6, 33.9, 31.7, 30.4, 29.2, 28.3, 23.5, 20.7, 20.0, 19.4, 17.1. MS (Q-TOF): m/z 552.4 (MH+).HRMS (Q-TOF): m/z 552.4017 (calculated (C30H54N3O6): 552.4013) X-ray powder diffraction: |
|
In ethanol; |
Example 1: Preparation of aliskiren hemifumarate 9 g of aliskiren were dissolved in 26 ml of ethanol and mixed with 0.95 g of fumaric acid. The mixture was filtered off and the solvent was evaporated. The residue was dissolved in 220 ml of acetonitrile and stirred at ambient temperature. The resulting solution was seeded with 50 mg of aliskiren hemifumarate and agitated at ambient temperature for 17 hours. The suspension was cooled to -10C and filtered off by suction after 1 hour. The residue was washed with 3 x 25 ml of acetonitrile and then dried in a vacuum oven at 30C for 1 hour. The aliskiren hemifumarate was obtained as white crystals (7.6 g). 1H-NMR (DMSO-d6) δ/ppm: 7.62 (t, 1H), 7.20 (s, 1H), 6.83-6.79 (m, 3H), 6.69 (dd, 1H), 6.35 (s, 1H), 3.97 (t, 2H), 3.71 (s, 3H), 3.46 (t, 2H), 3.28 (m, 1H), 3.23 (s, 3H), 3.18-3.08 (m, 2H), 2.57 (m, 1H), 2.46-2.25 (m, 3H), 1.92 (m, 2H), 1.79 (m, 1H), 1.69-1.52 (m, 3H), 1.41-1.21 (m, 3H), 1.03 (s, 6H), 0.85-0.76 (m, 12H).X-ray powder diffraction: A sample of the obtained aliskiren hemifumarate was measured by X-ray powder diffractometry. The XRD pattern was obtained on a Philips PW3040/60 X'Pert powder diffractometer using X'celerator detector at CuKα radiation, 1.54178 ?, 3<2θ<30. The obtained X-ray powder diffractogram is shown in Figure 1 and is characterized by the following peaks: No. Pos. [2Th.] d-spacing [A] Rel. Int. [%] 1 7.3 12.07 47 2 10.0 8.89 67 3 11.0 8.01 55 4 14.9 5.93 41 5 17.8 4.97 58 6 19.1 4.65 95 7 20.0 4.44 100 8 21.6 4.11 66FT-IR spectroscopy: FT-IR spectra of KBr discs prepared from the obtained aliskiren hemifumarate were recorded over a wave number range of 4000-400 cm-1 on a Perkin Elmer FT-IR spectrometer Spectrum GX at a resolution of 4 cm-1. The obtained FT-IR spectrum is shown in Figure 2.Thermal analysis: DSC thermograms were recorded on a DSC 822e Mettler Toledo scanning calorimeter. Samples of approx. 3 mg were scanned between 20C and 200C at a heating rate of 10C/min under nitrogen atmosphere. The obtained DSC thermogram is shown in Figure 3.Microscopy: Images of particles of the obtained aliskiren hemifumarate were taken on a Microscope Olympus BX 50 equipped with Olympus camera DP70. The sample was prepared as suspension of aliskiren hemifumarate in paraffinic oil. The obtained images are shown in Figures 4 and 5. |
|
In ethanol; at 25 - 30℃; for 0.166667h;Product distribution / selectivity; |
<strong>[173334-57-1]Aliskiren</strong> free base (50 gm) was dissolved in ethanol (150 ml) and stirred for 10 minutes. To this solution fumaric acid (5.2 gm) was added and stirred for 10 minutes at 25-30C. Filtered the reaction mixture and distilled off the solvent from the filtrate under reduced pressure and then co-distilled with dichloromethane to obtain the residue, n- pentane was added to the obtained residue, isolated the product in n-pentane and then dried to get aliskiren hemifumarate.Yield: 51.0 grams; purity by HPLC: 99%. |
|
In ethanol; acetonitrile; at 0 - 40℃; for 22h; |
The compound of Formula-Z (50g) was hydrogenated for 5-6 hours in the presence of 10% Pd/C (5 g) and ethanolic ammonia solution (10% w/w ammonia in ethanol ~29.4g) in ethanol (400 ml) at ambient temperature and 7 Kg/cm2 pressure. The reaction mixture was filtered and the catalyst was washed with ethanol (50 ml) and distilled to get residue. The obtained residue was co-distilled with acetonitrile (50 ml) and re-dissolved in ethanol and acetonitrile at 35-40C. To this fumaric acid (4.5g) was added and stirred to get clear solution and filtered at 35-40C to remove any insoluble particles. Acetonitrile (150 ml) was added to the above clear filtrate at 35-40C and inoculated with 200mg of <strong>[173334-57-1]Aliskiren</strong> hemifumarate and agitated for 3hours to get precipitation of the product. To the above slurry acetonitrile was added and agitated for 17 hours at ambient temperature. The suspension was cooled to 0 C and filtered off by suction after 2 hours. The product cake was washed with acetonitrile and then dried under vacuum at 35C to yield 47g of <strong>[173334-57-1]Aliskiren</strong> hemifumarate as white crystals. |
| 30 g |
In isopropyl alcohol; at 40℃; |
In a clean single-necked flask, 40 g of aliskiren free base and 4.2 g of fumaric acid and 320mL of isopropanol was added and heated to 40 C and dissolved, filtered while hot, The filtrate was slowly cooled to 3 C for static crystallization, and after about 10 hours a large amount of solid precipitated. Filtered, the solid was washed twice with a small amount of isopropyl alcohol, The solid was dried to give 30.0 g of a white powdery solid with an HPLC purity of 99.2% |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping