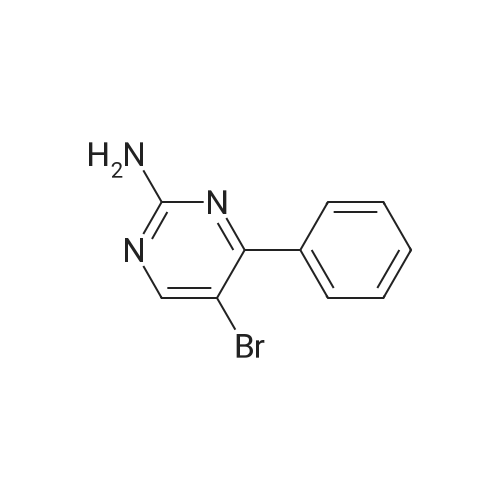
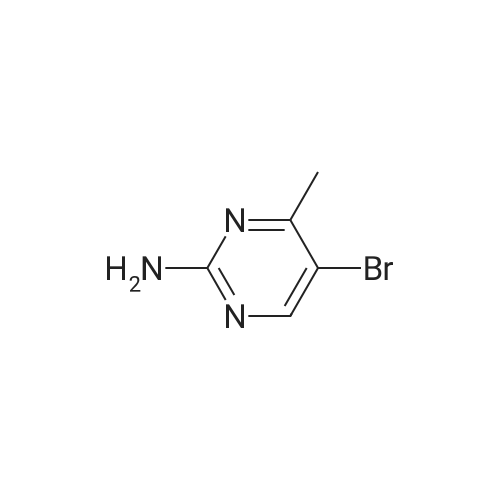
| 74% |
With potassium acetate In 1,4-dioxane for 8.33333 h; Inert atmosphere |
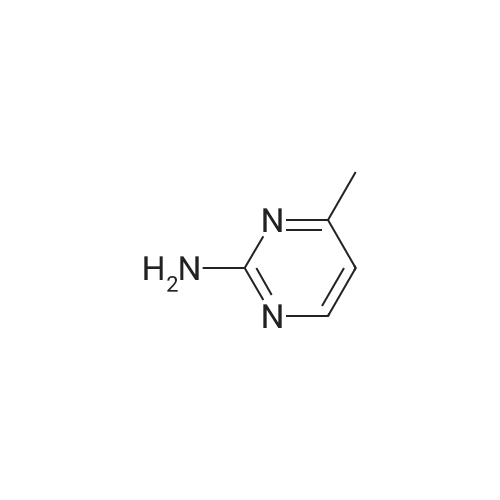
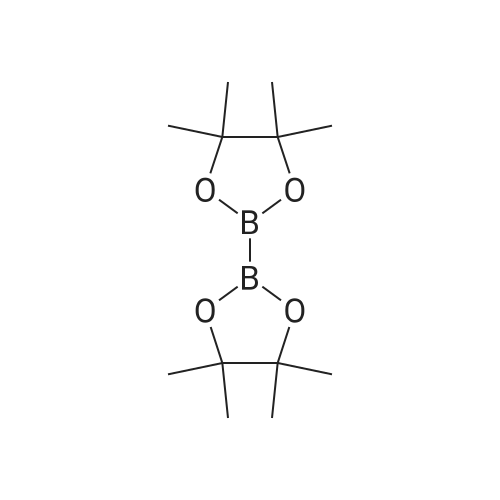
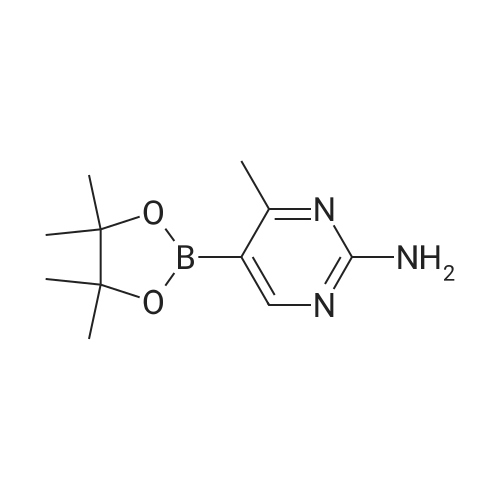
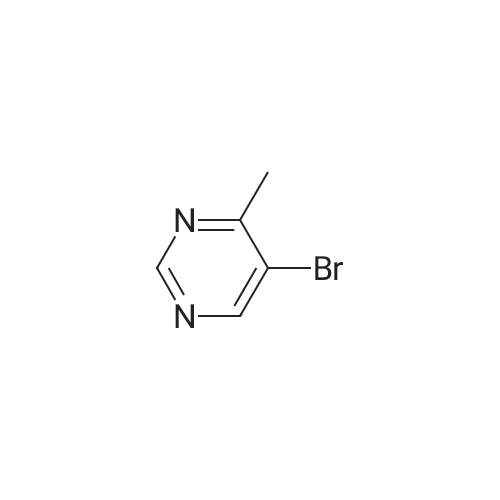
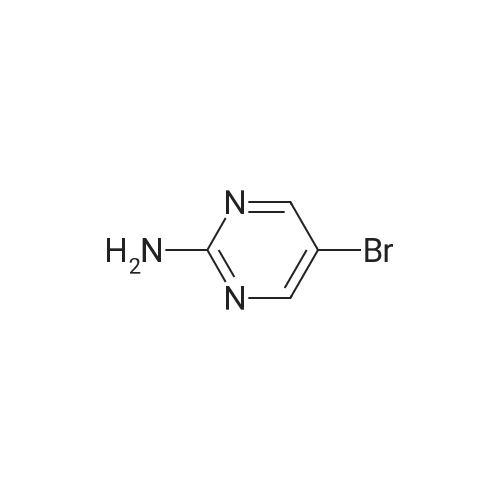
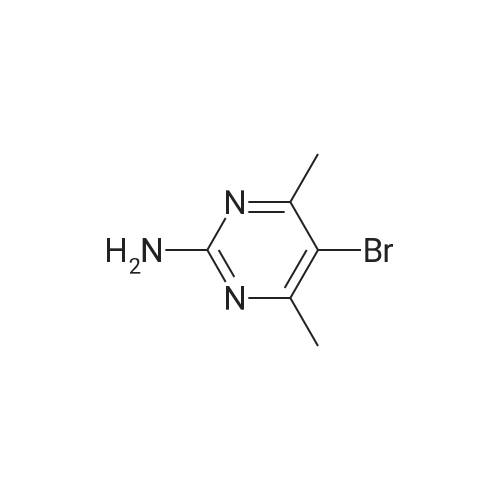
A mixture of 5-bromo-4-methylpyrimidine-2-ylamine (5.0g, 26 mmol), potassium acetate (7.83g, 79.8 mmol), 4,4,5,5-tetramethyl-2-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)- 1,3,2- dioxaborolane (7.43 g, 29.2 mmol) in dioxane (140 mL) was stirred for 20 min under nitrogen. 1, 1 '-bis (diphenylphosphino) ferrocene palladium (II) chloride dichloromethane adduct (1.08 g, 1.33 mmol) was added to the reaction mixture. The reaction mixture was heated to 115 °C for 18 h under nitrogen. Upon completion, the mixture was cooled and EtOAc was added. The resulting mixture was sonicated and filtered. Additional EtOAc was used to wash the solid. The combined organic extracts were washed with water, dried over MgS04, filtered and concentrated. The crude was purified by chromatography eluting with 20-100percent EtO Ac/hex ane to yield 4.5 g of 4-methyl-5-(4,4,5,5-tetramethyl (l,3,2-dioxaborolan-2-yl))pyrimidine-2-ylamine (yield: 74percent). 1H-NMR (DMSO, 400 MHz): δ 8.28 (s, 1H), 6.86 (br s, 2H), 2.35 (s, 3 H), 1.25 (s, 12 H). MS (ESI) m/e (M+H+) 236.15, 154.07. |
| 74% |
With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; potassium acetate In 1,4-dioxane at 115℃; for 18 h; Inert atmosphere |
A mixture of 5-bromo-4-methylpyrimidine-2-ylamine (5.0 g, 26 mmol), potassium acetate (7.83 g, 79.8mmol), 4,4,5,5-tetramethyl-2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1,3,2-dioxaborolane (7.43 g,29.2 mmol) in dioxane (140 mL) was stirred for 20 min under nitrogen. 1,1′-bis(diphenylphosphino)ferrocene palladium (II) chloride dichloromethane adduct (1.08 g, 1.33 mmol) was added to the reaction mixture. The reaction mixture was heated to 115° C. for 18 h under nitrogen. Upon completion, the mixture was cooled and EtOAc was added. The resulting mixture was sonicated and filtered. Additional EtOAc was used to wash the solid. The combined organic extracts were washed with water, dried over MgSO4, filtered and concentrated. The crude was purified by chromatography eluting with 20~100percent EtOAc/hexane to yield 4.5 g of 4-methyl-5-(4,4,5,5-tetramethyl (1,3,2-dioxaborolan-2-yl))pyrimidine-2-ylamine 26 (yield: 74percent). 1H-NMR (DMSO, 400 MHz): δ 8.28 (s, 1H), 6.86 (br s, 2H), 2.35 (s, 3H), 1.25 (s, 12H). MS (ESI) m/e (M+H+) 236.15, 154.07. |
| 74% |
With potassium acetate In 1,4-dioxane at 115℃; for 18 h; Inert atmosphere |
A mixture of 5-bromo-4-methylpyrimidine-2-ylamine (5.Og, 26 mmol ), potassium acetate (7.83g, 79.8 mmol), 4,4,5,5-tetramethyl-2-(4,4,5,5-tetramethyl-l,3,2- dioxaborolan-2-yl)- 1,3,2-dioxaborolane (7.43 g, 29.2 mmol) in dioxane (140 mL) was stirred for 20 min under nitrogen. 1, l'-bis (diphenylphosphino) ferrocene palladium (II) chloride dichloromethane adduct (1.08 g, 1.33 mmol) was added to the reaction mixture. The reaction mixture was heated to 115 ° C for 18 h under nitrogen. Upon completion, the mixture was cooled and EtOAc was added. The resulting mixture was sonicated and filtered. Additional EtOAc was used to wash the solid. The combined organic extracts were washed with water, dried over MgSO4, filtered and concentrated. The crude was purified by chromatography eluting with 20-100percent EtOAc/hexane to yield 4.5 g of 42 (yield: 74percent). 1H-NMR (DMSO, <n="71"/>400 MHz): δ 8.28 (s, IH), 6.86 (br s, 2H), 2.35 (s, 3 H), 1.25 (s, 12 H). MS (ESI) m/e (M+H+) 236.15, 154.07. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping