| 81% |
With hydrogen;palladium 10% on activated carbon; In ethanol; at 35 - 57℃; under 2327.23 Torr; for 4h;Parr reactor;Product distribution / selectivity; |
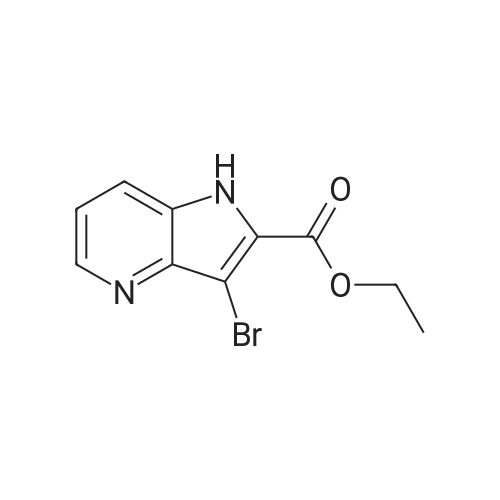
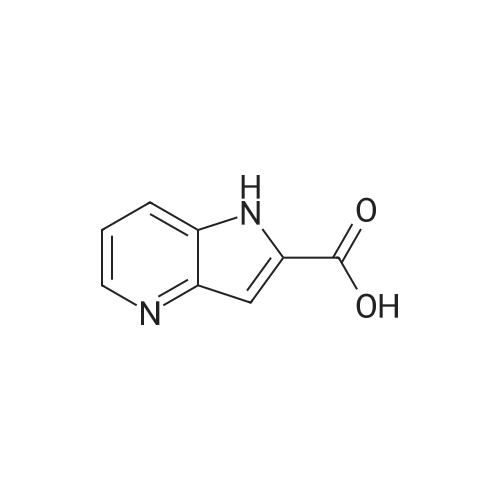
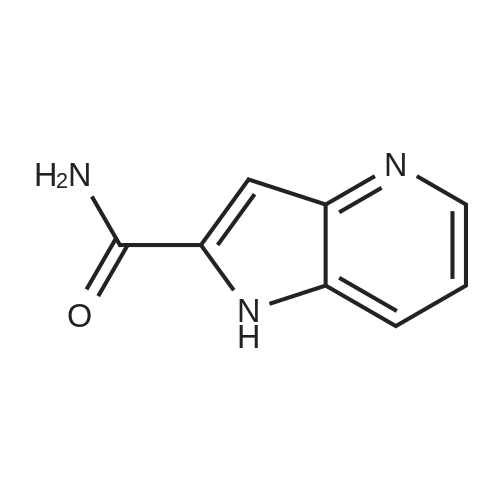
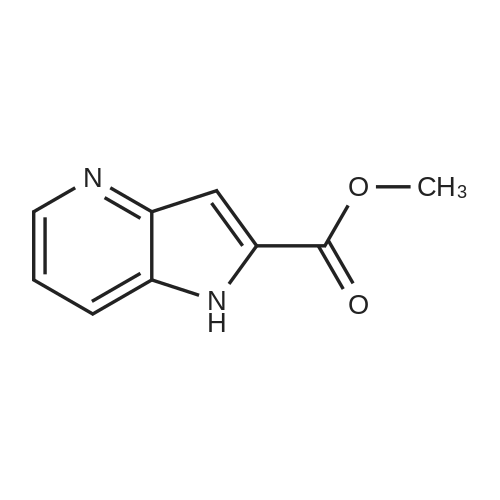
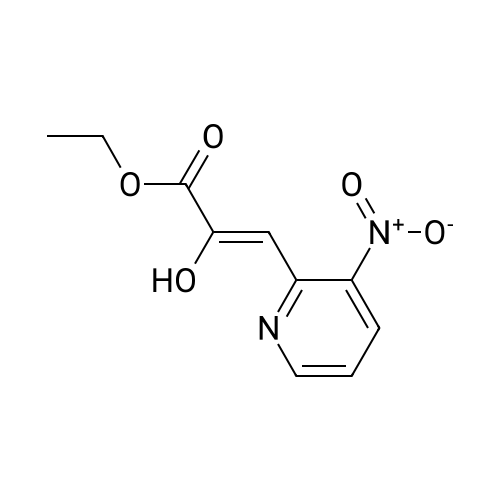
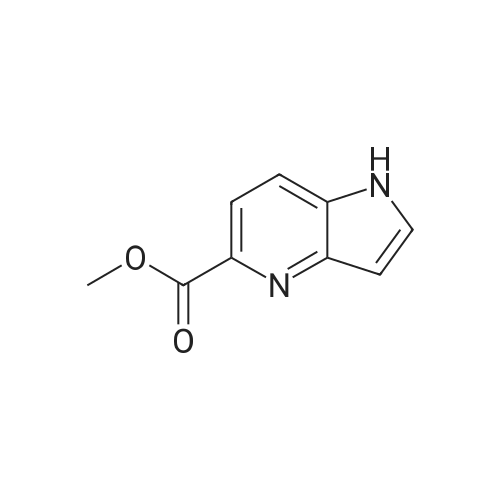
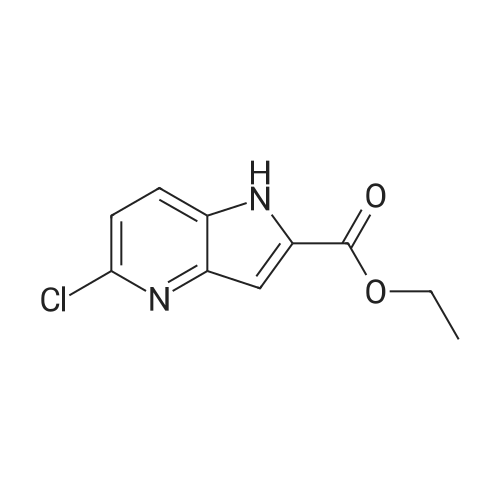
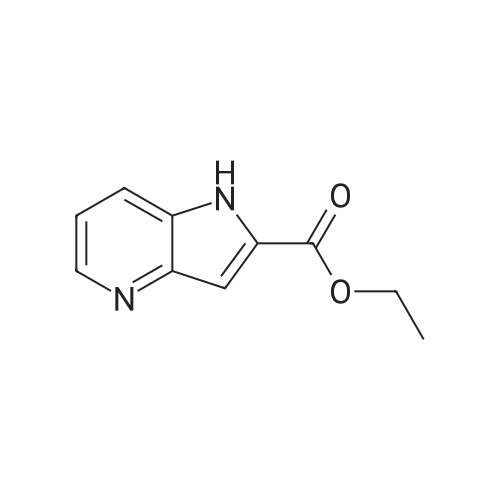
1H-Pyrrolo[3,2-b]pyridine-2-carboxylic acid ethyl ester, 4a-1 (Scheme 1, step c) Add to a 2 L thick-walled Parr reactor compound 3a-1 (56.8 g, 0.24 mol), ethanol (200 proof, 850 mL, 15 parts) and 10percent Pd/C (5.7 g, 10percent by wt.). Connect the reaction vessel to a Parr hydrogenator, flush with hydrogen and pressurize the orange slurry to 45 psi. Shake at rt for 1 h during which time the temperature rises to 57° C. When the temperature of the reaction mixture stabilizes at 35° C., slowly heat the reaction to 40° C. for 3 h. When the reaction is complete as determined by-TLC (silica gel, 1percent MeOH in CH2Cl2), cool the reaction mixture to rt, filter the slurry through Celite.(R)., and wash the filter cake with EtOH (4*200 mL). Concentrate the yellow filtrate to afford a solid (41.6 g), add ethyl acetate (302 mL) and heat on a steam bath. Cool the mixture to rt and add heptane (600 mL) to precipitate the product. Stir the mixture in an ice bath for 1 h, filter and wash the filter cake with heptane (100 mL). Dry the filter cake (50° C./0.1 in Hg) for 24 h to give 4a-1 as a light gray solid (36.6 g, 81percent yield). 1H NMR (DMSO-d6) 1.36 (t, 3H, J=7.0 Hz), 4.36 (q, 2H, J=7.0 Hz), 7.19 (s, 1H), 7.25 (dd, 1H, J=4.5, 8.1 Hz), 7.82 (d, 1H, J=8.1 Hz), 8.44 (d, 1H, J=4.5 Hz), delta 12.11 (s, 1H). |
| 81% |
With titanium tetrachloride; tin(ll) chloride; In ethanol; for 4h;Heating / reflux;Product distribution / selectivity; |
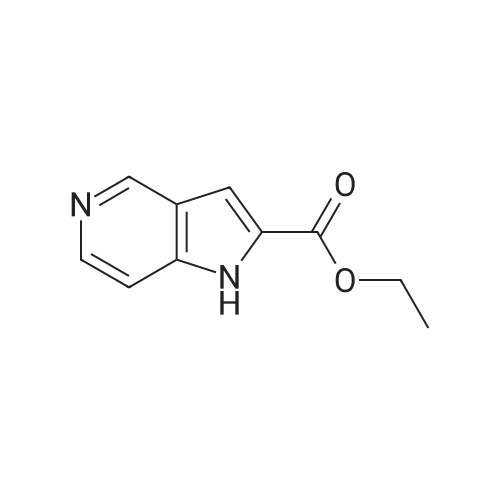
Hydrogenate 3a-1 (20 g) over 10percent palladium on carbon (5.5 g) in EtOH (350 mL) at rt for 3 hours under 1200 psi. Filter the reaction mixture through Celite.(R). and concentrate the filtrate to give ester 4a-1 (R4H) in 39percent yield. Alternatively, reduce 3a-1 with SnCl2 (5.0 equiv), TiCl4 (2.5 equiv) in EtOH at reflux for 4 h, cool to ambient temperature, concentrate and purify by silica gel chromatography to provide ester 4a-1 (R4H) in 81percent yield. 1H NMR (DMSO-d6) delta 13.48 (bs, 1H), 8.80 (dd, 1H, J=0.7, 5.4 Hz), 8.56 (dd, 1H, J=0.7, 8.4 Hz), 7.80 (dd, 1H, J=5.5, 8.4 Hz), 7.37 (s, 1H), 4.40 (q, 2H, J=7.0 Hz), 1.38 (t, 3H, J=7.0 Hz). |
| 39% |
With hydrogen;palladium 10% on activated carbon; In ethanol; at 20℃; under 62059.4 Torr; for 3h;Product distribution / selectivity; |
Hydrogenate 3a-1 (20 g) over 10percent palladium on carbon (5.5 g) in EtOH (350 mL) at rt for 3 hours under 1200 psi. Filter the reaction mixture through Celite.(R). and concentrate the filtrate to give ester 4a-1 (R4H) in 39percent yield. Alternatively, reduce 3a-1 with SnCl2 (5.0 equiv), TiCl4 (2.5 equiv) in EtOH at reflux for 4 h, cool to ambient temperature, concentrate and purify by silica gel chromatography to provide ester 4a-1 (R4H) in 81percent yield. 1H NMR (DMSO-d6) delta 13.48 (bs, 1H), 8.80 (dd, 1H, J=0.7, 5.4 Hz), 8.56 (dd, 1H, J=0.7, 8.4 Hz), 7.80 (dd, 1H, J=5.5, 8.4 Hz), 7.37 (s, 1H), 4.40 (q, 2H, J=7.0 Hz), 1.38 (t, 3H, J=7.0 Hz). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping