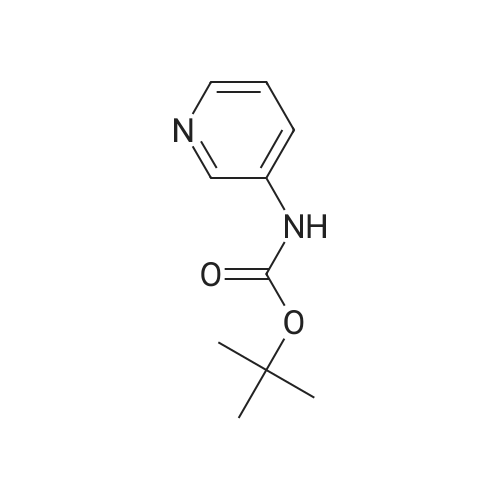
| 32.3% |
|
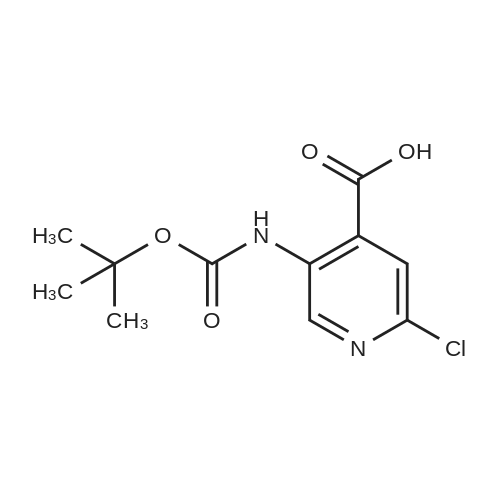
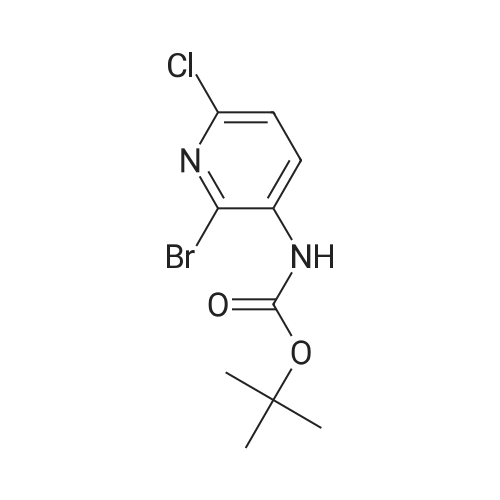
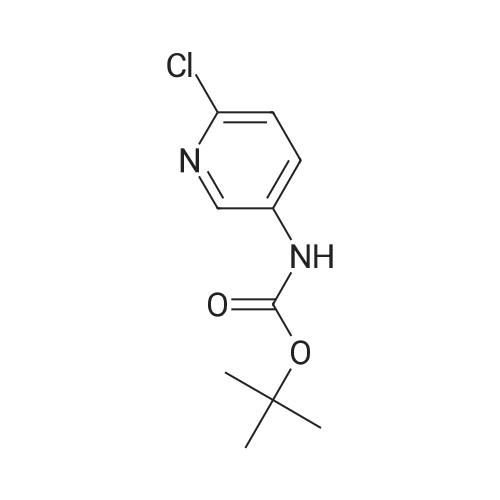
To the solution of tert-butyl (6-chloropyridin-3-yl)carbamate (160 g, 0.7 mol) in 1 L of anhydrous THF was added n-BuLi (600 mL, 1.5 mol) at -78 C. under N2atmosphere. After the addition was finished, the solution was stirred at -78 C. for 30 min, and the solution of I2(177.68 g, 0.7 mol) in 800 mL of anhydrous THF was added. Then the solution was stirred at -78 C. for 4 hrs. TLC indicated the reaction was over. Water was added for quench, and EtOAc was added to extract twice. The combined organic phases were washed with brine, dried over Na2SO4, filtered and purified by flash chromatography to afford 80 g of tert-butyl (6-chloro-4-iodopyridin-3-yl)carbamate as a yellow solid (32.3%). |
|
|
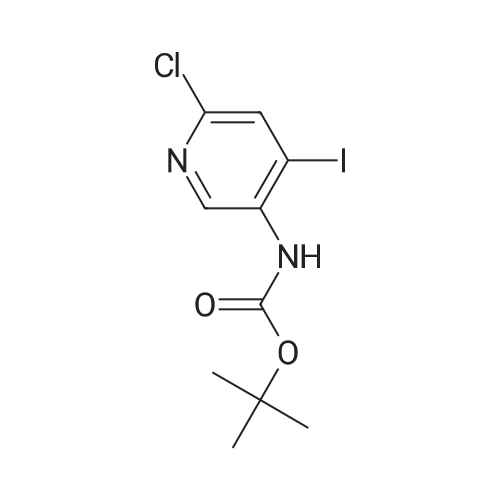
Preparation 7 : (6-Chloro-4-iodopyridin-3-yl)carbamic acid tert-butyl ester The title compound was prepared according to the method described in US 2002/0022624 Al from the compound of Preparation 6. 5H(CDC13): 1.54 (9H, s), 6.62 (IH, s), 7.72 (IH, s), 8.93 (IH, s). |
|
|
Step B: tert-Butyl (6-chloro-4-iodopyridin-3-yl)carbamateteri-Butyllithium (1.3 M in heptanes, 1 11 mL, 144 mmol) was added dropwise to a solution of t-butyl (6-chloropyridin-3-yl)carbamate (15.0 g, 65.6 mmol) in anhydrous THF (300 mL) at -78 C over 30 min under 2 atmosphere. The resulting mixture was stirred at -78 C for 1 h, then at -10 C for 1 h. The reaction mixture was cooled to -78 C and a solution of I2 (36.6 g, 144 mmol) in anhydrous THF (100 mL) was added. The resulting mixture was warmed to ambient temperature and stirred for 18 h. Excess ?-butyllithium and I2 were quenched with saturated aqueous NH4C1 solution (150 mL) and saturated aqueous a2S203 solution (500 mL), respectively, and the resulting mixture was stirred for 30 min. The organic layer was separated and the aqueous layer was extracted with EtOAc (250 mL x 2). The combined organic layers were washed with brine (250 mL), dried over Na2S04 and concentrated. The residue was purified by column chromatography on silica gel (EtOAc:PE:Et3N = 2 : 98 : 1, then 2.5 : 97.5 : 1) to afford the title compound. MS: m/z = 355 (M + 1). XH NMR (400 MHz, CDC13) delta 8.93 (s, 1H), 7.72 (s, 1H), 6.64 (s, 1H), 1.53 (s, 9H). |
| 2.59 g |
|
Under argon gas atmosphere, to diethyl ether (120mL)solution of (6-chloropyridin-3-yl) carbamic acid tert- butyl (5.0 g) and N, N,N ', N'- tetramethyl ethane-1,2-diamine (7.7 g), at -78 n-butyl lithium solution (2.65mol / L tetrahydrofuran solution, 25mL) was addeddropwise. after stirring the mixture for 2 hours at -10 , diethyl ether (40mL) solution of iodine (11.4g) wasdropped at -78 , and the mixture was stirred one day at roomtemperature. To the reaction mixture, a saturated aqueous ammonium chloridesolution was added, and extracted with diethyl ether. The organic layer was washed with 10%aqueous solution of sodium pyrosulfite and saturated brine, dried overanhydrous magnesium sulfate, and evaporated under reduced pressure. Theresulting crude product was purified by silica gel column chromatography (eluting solvent: n- hexane / ethyl acetate = 100 / 0 ~60 / 40) to give the title compound (2.59 g). |
| 2.59 g |
|
To a solution of (6-chloropyridin-3-yl)carbamic acid tert-butyl ester(5.0 g) at -78 C and N, N, N ', N'-tetramethylethane-1,2-diamine (7.7 g) of diethyl ether (120 mL) after the drop of the solution was stirred at 10 C for 2 hours, iodine (11.4 g) was added dropwise at -78 C, of diethyl ether (40 mL) solution. The mixture was stirred at room temperature for one day. To the reaction mixture was added a saturated aqueous ammonium chloride solution, and extracted with diethyl ether. The organic layer was washed with 10% aqueous sodium metabisulfite solution, after washing with saturated saline, and then dried over anhydrous magnesium sulfate, and distilled off under reduced pressure. The obtained crude product was purified by silica gel column chromatography (elution solvent; n-hexane-ethyl acetate) to give the title compound (2.59 g). |
| 2.59 g |
|
Reference Example 4 (0080) (6-Chloro-4-iodopyridin-3-yl)cathamic acid tert-butyl ester (0081) To a solution of (6-chloropyridin-3-yl)cathamic acid tert-butyl ester (5.0 g) and N,N,N?,N-tetramethylethane-1,2-diamine (7.7 g) in diethyl ether (120 mL) was added dropwise n-buthyllithium (2.65 mol/L n-hexane solution, 25 mL) at -78 C. under an argon gas atmosphere. After the mixture was stirred at -10 C. for 2 hours, a solution of iodine (11.4 g) in diethyl ether (40 mL) was added dropwise at -78 C. The resulting mixture was stirred at room temperature for 1 day. To the reaction mixture was added a saturated aqueous ammonium chloride solution, and the resulting mixture was extracted with diethyl ether. The organic layer was washed with 10% aqueous sodium pyrosulfite and brine, and dried over anhydrous magnesium sulfate, and the solvent was removed under reduced pressure. The obtained crude product was purified by column chromatography on silica gel (eluent: n-hexane/ethyl acetate) to give the title compound (2.59 g). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping