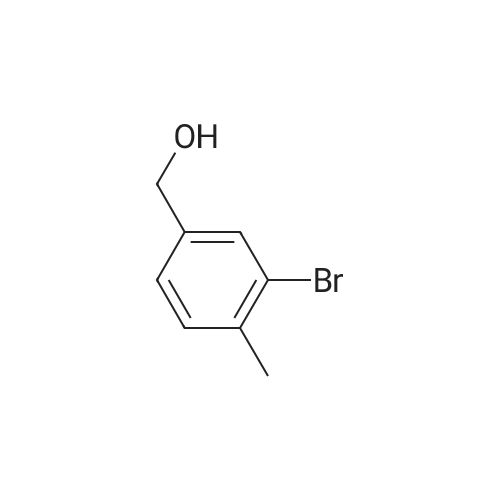
| 89% |
With phosphorus tribromide; In dichloromethane; at 0 - 20℃; for 1h; |
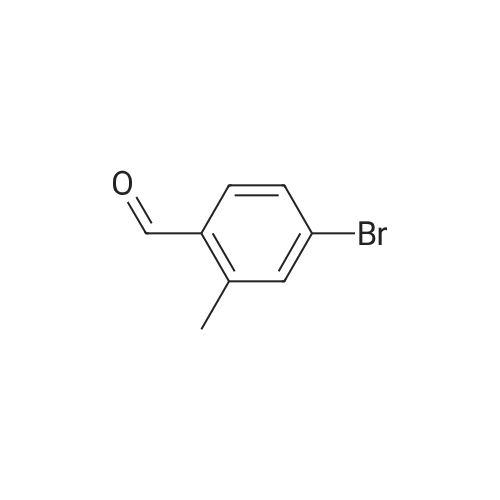
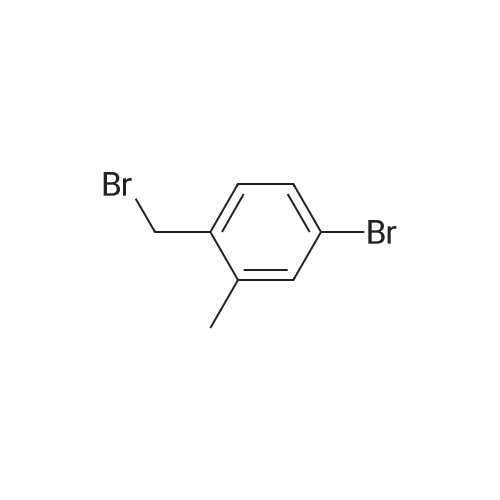
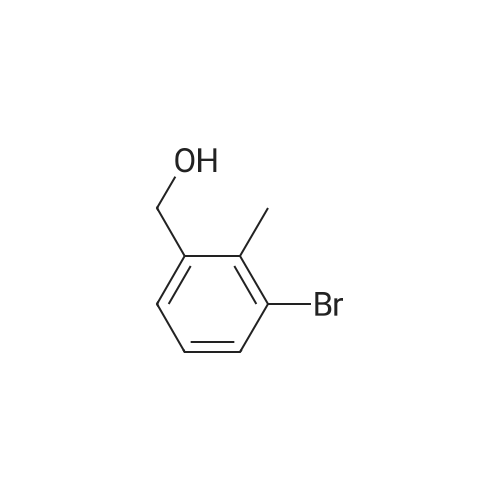
[0653j To a solution of (4-bromo-2-methylphenyl)methanol (1.3 g, 6.7 mmol) in CH2C12 (50 mL) was added PBr3 (0.95 mL, 10 mmol) at 0 C. The mixture was stirred at rt for 1 h, quenched with ice-water (50 mL) and the pH value was adjusted to 7.0 with 50% aqueous NaOH solution. The mixture was extracted with EtOAc (100 mL x 2) and the combined organic layers were washed with water (50 mL), dried (Na2SO4) and concentrated in vacuo to give 4-bromo-1- (bromomethyl)-2-methylbenzene (1.56 g, yield: 89%) as a white solid which was used in next step without further purification. |
| 81% |
With carbon tetrabromide; triphenylphosphine; In dichloromethane; at 20℃; for 5h; |
Triphenylphosphine (11.35 g, 43.27 mmol) followed by carbon tetrabromide (14.35 g, 43.27 mmol) were added to a solution of (4-bromo-2-methyl-phenyl)-methanol (7.25 g, 36 mmol) in CH2CI2 (200 mL). The mixture was stirred at room temperature for 5 hours. The solution was concentrated to 15 ml. The residue was purified by flash column chromotography (1% to 10% EtOAc in Hexane) gave the product as brown oil (9.25 g, 81% yield). 1H NMR (400 MHz, CDCI3) 5: 2.39 (3 H, s) 4.45 (2 H, s) 7.17 (1 H, d, J=8.08 Hz) 7.29 (2 H, m) 7.30 (1 H, dd, J=8.08, 2.02 Hz) 7.34 (1 H, s) |
|
With N-Bromosuccinimide; triphenylphosphine; In dichloromethane; |
4-Bromo-1-bromomethyl-2-methyl-benzene (Intermediate 138) A solution of (4-bromo-2-methyl-phenyl)-methanol (Intermediate 133, 319.0 mg, 1.58 mmol) and triphenylphosphine (466.0 mg, 1.74 mmol) in 5 mL CH2Cl2 was cooled to 0 C. and N-bromosuccinimide (309.0 mg, 1.74 mmol) was added in 5 portions over 20 minutes. The solution was warmed to 25 C. and stirred for 17 hours. The reaction was quenched by the addition of dilute aqueous NaHCO3. The resulting mixture was extracted with Et2O and the combined organic layers were washed with H2O and saturated aqueous NaCl before being dried (Na2SO4) and concentrated under reduced pressure. The title compound, 350.0 mg (84%), was isolated by column chromatography (2-3% EtOAc-hexanes) as a colorless oil. 1H NMR (CDCl3) delta: 7.32 (1H, d, J=2.0 Hz), 7.29 (1H, dd, J=2.0, 7.9 Hz), 7.15 (1H, d, J=7.9 Hz), 4.43 (2H, s), 2.37 (3H, s). |
|
With triphenylphosphine; In N-Bromosuccinimide; |
4-Bromo-1-bromomethyl-2-methyl-benzene (Intermediate 138) A solution of (4-bromo-2-methyl-phenyl)-methanol (Intermediate 133, 319.0 mg, 1.58 mmol) and triphenylphosphine (466.0 mg, 1.74 mmol) in 5 mL CH2C12 was cooled to 0 C. and N-bromosuccinimide (309.0 mg, 1.74 mmol) was added in 5 portions over 20 minutes. The solution was warmed to 25 C. and stirred for 17 hours. The reaction was quenched by the addition of dilute aqueous NaHCO3. The resulting mixture was extracted with Et2O and the combined organic layers were washed with H2O and saturated aqueous NaCl before being dried (Na2SO4) and concentrated under reduced pressure. The title compound, 350.0 mg (84%), was isolated by column chromatography (2-3% EtOAc-hexanes) as a colorless oil. 1H NMR (CDCl3) delta: 7.32 (1H, d, J=2.0 Hz), 7.29 (1H, dd, J=2.0, 7.9 Hz), 7.15 (1H, d, J=7.9 Hz), 4.43 (2H, s), 2.37 (3H, s). |
|
With carbon tetrabromide; triphenylphosphine; In dichloromethane; at 20℃; |
To a stirred solution of (4-Bromo-2-methyl-phenyl)-methanol (as obtained in preparation 25) (4.58 g, 22.8 mmol) in CH2CI2 (50 ml) is added , under Argon and at RT, carbon tetrabromide (9.27 g, 27.4 mmol) followed by thphenylphosphine (7.25 g, 27.4 mmol). The mixture is stirred overnight and then concentrated in vacuo. The crude residue is then purified by chromatography (silicagel, hexane: EtOAc 25:1) to afford the title compound as a clear oil. |
|
With hydrogen bromide; acetic acid; In water; at 50℃; for 12h; |
Step 2: 4-bromo-l-(bromomethyl)-2-methylbenzene; Hydrobromic acid (cone, 2 eq.) was added to a stirred solution of the alcohol from step 1 (1 eq.) in acetic acid (0.22M). The mixture was stirred at 50 C for 12 h, cooled down to room temperature, poured in water and extracted with Et2O. The organic extract was washed with <n="25"/>water, aqueous sodium hydrogen carbonate (3x), brine, dried over MgSO4, filtered and concentrated to afford the desired benzyl bromide as a light yellow solid. |
|
With phosphorus tribromide; In dichloromethane; at 0 - 20℃; for 3h; |
To a solution of (4-bromo-2-methylphenyl)methanol 132 (4 g) in DCM was added PBr3 (5.42 g) at 0 C. The mixture was stirred for 3 h at room temperature. DCM was added and washed with water and aq NaHC03 until pH ~ 7. The organic layer was separated, dried over Na2S04, and concentrated under reduced pressure to give 4-bromo- l -(bromomethyl)-2- methylbenzene 133 as a brown oil (3.8 g). The compound was used in the next reaction without further purification. ? NMR (400 MHz, CDC13) ppm: 7.37 (s, 1 H), 7.34 (d, J= 8 Hz, 1 H), 7.20 (d, J= 8 Hz, 1 H), 4.48 (s, 2H), 2.41 (s, 3H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping