Alternatived Products of [ 17082-47-2 ]
Product Details of [ 17082-47-2 ]
| CAS No. : | 17082-47-2 |
MDL No. : | MFCD00053848 |
| Formula : |
C8H28O4Si5
|
Boiling Point : |
No data available |
| Linear Structure Formula : | - |
InChI Key : | UOUILILVWRHZSH-UHFFFAOYSA-N |
| M.W : |
328.73
|
Pubchem ID : | 11056564 |
| Synonyms : |
|
Application In Synthesis of [ 17082-47-2 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 17082-47-2 ]
- 1
-
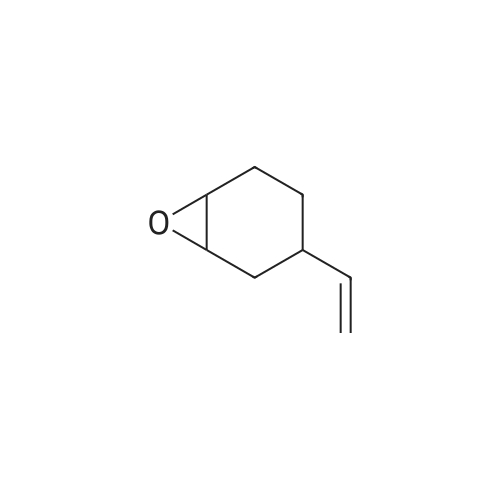 [ 106-86-5 ]
[ 106-86-5 ]

-
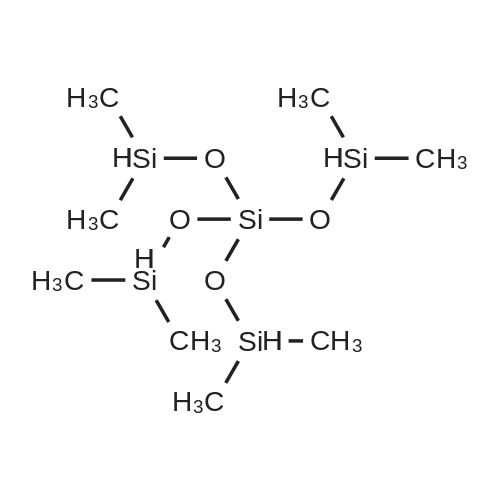 [ 17082-47-2 ]
[ 17082-47-2 ]

-
 [ 121239-70-1 ]
[ 121239-70-1 ]
| Yield | Reaction Conditions | Operation in experiment |
|
platinum(II)Cl2(bis(triphenylphsphine)); In dichloromethane; toluene; at 129 - 140℃; for 3h;Heating / reflux; |
This example describes the preparation of tetrakis(1,2-epoxy-4-cyclohexylethyl dimethylsiloxy)silane, ME4Q (represented by formula (VI)) using parts per billion levels of PtCl2(PPh3)2 as the hydrosilation catalyst. A 1 liter, 3-necked round-bottomed flask equipped with two condensers and a thermometer was charged with VCHO (121.3 g, 977 mmol), 66.1 g (201 mmol) of MH4Q (tetrakis(dimethylsilyloxy)silane), 4.4 microliters of PtCl2(PPh3)2 catalyst solution (140 ppb Pt based on final product weight), and 80 g toluene.. The reaction solution was heated to reflux with stirring, resulting in a solution temperature of 129C. At this point the reaction mixture began to reflux vigorously, with the solution temperature reaching 140C initially, and then settling down to 132C. The mixture was then refluxed for 3 hours.. At this point, the infrared spectrum of an aliquot of the reaction mixture showed no Si-H absorption indicating complete consumption of MH4Q. The solution was cooled to room temperature, and the condensers and thermometer were replaced with stopcocks and a short-path distillation head. The solution was heated with a water bath at 85C and stripped of volatile components until the vapor temperature remained above 50C for about 30 min.. No gel formation was observed during and after the stripping process.. Proton NMR spectrum of the residual material in the reaction flask showed it to be the desired product and free of residual solvent and VCHO. In this manner, 160 g of (VI) was obtained as a colorless material. The results obtained in the above Examples are summarized in Table 1 below.. Examples 1 - 3 demonstrate that organosilicon compositions without any gel formation may be prepared according to the method of the present invention using less than 10 percent of the catalyst employed in conventional methods of preparing organosilicon compositions. For completeness, various aspects of the invention are now set out in the following numbered clauses: |
- 2
-
 [ 106-86-5 ]
[ 106-86-5 ]

-
 [ 17082-47-2 ]
[ 17082-47-2 ]

-
C24H52O6Si5
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
polymer-bound Wilkinson's catalyst; |
The new synthetic methodology described above was also applied to the preparation of several epoxy-functional oligomeric siloxanes. For example, shown above in Scheme 5 is a method for the preparation of an epoxy functional oligomeric silicone that bears pairs of reactive epoxycyclohexyl groups disposed along the backbone of the chain on every third silicon atom. The initial hydrosilation reaction of tetrakis (dimethylsiloxy) silane with two equivalents of 3-vinyl-7-oxabicyclo [4.1. 0] heptane proceeded smoothly to yield an intermediate whose'H-NMR spectrum is consistent with a structure, XIIA-C, possessing two epoxycyclohexyl groups and two Si-H moieties. The intermediate was not isolated but directly subjected to a rhodium catalyzed dehydrodimerization reaction. Condensation proceeded slowly at 80-100 C in the presence of oxygen, and oligomers with different degrees of polymerization were formed. Similarly, in Scheme 6 is depicted the synthesis of an oligomer in which cyclic siloxane units bearing pendant epoxycyclohexyl groups are situated along the backbone. Although the symmetrical disubstituted intermediate XIV is shown leading to an oligomer with structure XV, it is acknowledged that the presence of other isomeric structures is also probable. Replacement of the epoxycyclohexyl groups with other types of epoxy, oxetane or vinyl ether groups can lead to still other classes of reactive oligomers. |
- 3
-
 [ 17082-47-2 ]
[ 17082-47-2 ]

-
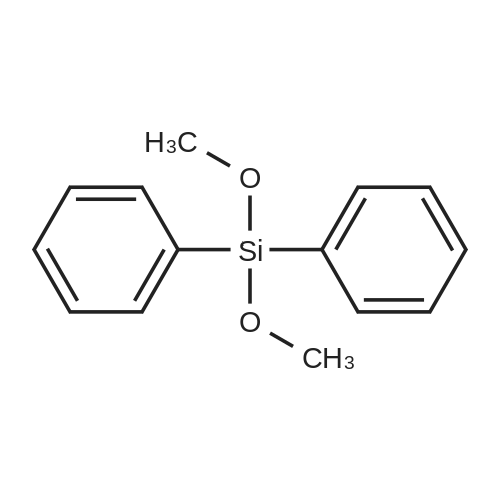 [ 6843-66-9 ]
[ 6843-66-9 ]

-
C32H44O8Si7
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 80% |
With tris(pentafluorophenyl)borate; In cyclohexane; for 1h; |
Into a 200 ml three-mouth flask was first added first 20 ml of cyclohexane and tris(pentafluorophenyl)borane (1.4 mg), then in another 25 ml container add tetrakis(dimethylsiloxy)silane (0.41 g, 1.25 mmol), <strong>[6843-66-9]<strong>[6843-66-9]diphenyldimethoxysilan</strong>e</strong> (0.611 g, 2.5 mmol) and 5 ml of cyclohexane. After mixing, suction into a 50 ml needle tube, slowly dripped into tris(pentafluorophenyl)borane in cyclohexane solution, bubbles immediately released and continuing throughout the dripping process, the whole dropping process lasts 1 hour. After the reaction, steaming and remove the solvent, then petroleum ether/dichloromethane mixture (10:1 ratio) is separated by silica gel chromatography eluting agent 0.753 g of white powder solid, yield is 80%. |
- 4
-
 [ 17082-47-2 ]
[ 17082-47-2 ]

-
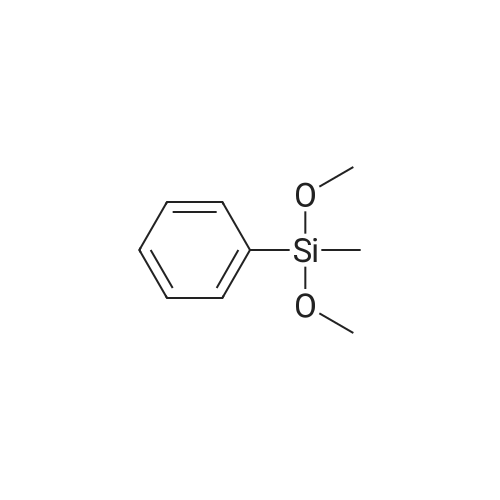 [ 3027-21-2 ]
[ 3027-21-2 ]

-
C22H40O8Si7
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 75% |
With tris(pentafluorophenyl)borate; In cyclohexane; for 1h; |
Into a 200 ml three-mouth flask was first added 20 ml of cyclohexane and tris(pentafluorophenyl)borane (1.4 mg), then in another 25 ml container tetrakis(dimethylsiloxy)silane (0.41 g, 1.25 mmol), phenyl(methyl)dimethoxysilane (0.455 g, 2.5 mmol) and 5 ml of cyclohexane. After mixing, suction into a 50 ml in the needle tube, slowly dripped into the tris(pentafluorophenyl)borane in cyclohexane solution, bubbles immediately released and continuing throughout the dripping process, the whole dropping process lasts 1 hour. After the reaction, steaming and remove the solvent, then petroleum ether/dichloromethane mixture (10:1 ratio) is separated by silica gel chromatography eluting agent 0.589 g of white powder solid, yield is 75percent. |
- 5
-
 [ 17082-47-2 ]
[ 17082-47-2 ]

-
 [ 3027-21-2 ]
[ 3027-21-2 ]

-
C15H34O6Si6
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 70% |
With tris(pentafluorophenyl)borate; In cyclohexane; at 20℃; for 0.333333h; |
Tetrakis(dimethylsiloxy)silane (CAS 17082-47-2) (0.65 g, 2 mmol), phenylmethyldimethoxysilane (CAS) was added to a 200 mL round bottom flask. 3027-21-2) (0.364 g, 2 mmol) and 20 mL of cyclohexane, then added a magnet, and stirred at room temperature. Tris (pentafluorophenyl) borane (CAS 1109-15-5) (1 mg) was added to the above solution, followed by stirring at room temperature for 20 minutes, followed by adding 0.3 g of activated carbon to the reaction solution, which was then filtered and collected filtrate. After the solvent was removed from the collected solution by a rotary evaporator, a liquid mixture was obtained, and then 0.640 g of a colorless liquid was isolated by distillation under reduced pressure in a yield of 70percent. The boiling point is 100°C/1.5kPa. |
- 6
-
 [ 17082-47-2 ]
[ 17082-47-2 ]

-
 [ 6843-66-9 ]
[ 6843-66-9 ]

-
C20H36O6Si6
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 70% |
With tris(pentafluorophenyl)borate; In cyclohexane; at 20℃; for 0.333333h; |
tetrakis(Dimethylsiloxy) silane (CAS 17082-47-2) (0.657 g, 2 mmol), <strong>[6843-66-9]<strong>[6843-66-9]diphenyldimethoxysilan</strong>e</strong> (CAS 6843-66 -9) (0.489 g, 2 mmol) and 20 mL of cyclohexane, then added a magnet and stirred at room temperature. Tris(pentafluorophenyl)borane (CAS 1109-15-5) (1 mg) was added to the foregoing solution, followed by stirring at room temperature for 20 minutes, followed by addition of 0.3 g of activated carbon to the reaction solution, followed by filtration and collection. filtrate. The collected solution was solvent-removed on a rotary evaporator to give a liquid mixture, and then 0.757 g of a colorless liquid was isolated by distillation under reduced pressure with a yield of 70%.The boiling point is 120C/1.5kPa. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping









