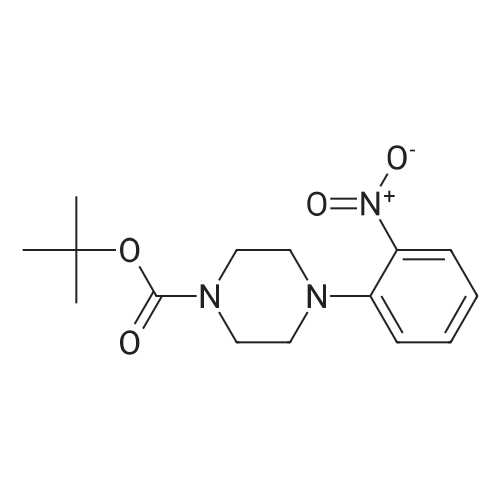
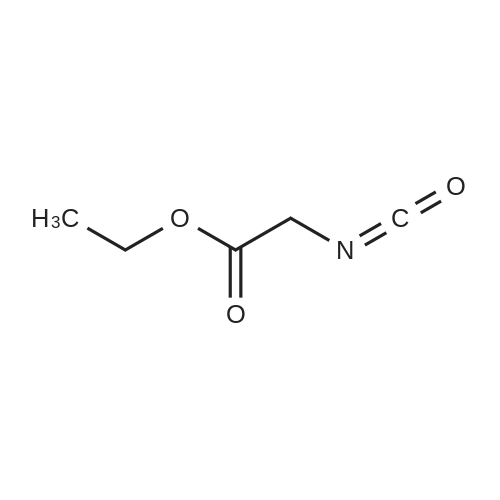
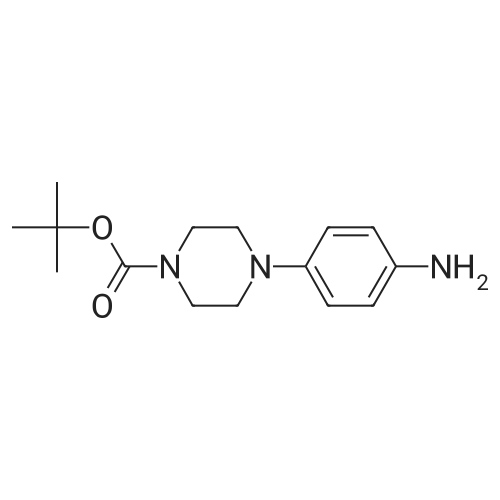
| 89% |
With ethanol; iron; ammonium chloride; In water; at 90℃; for 2h; |
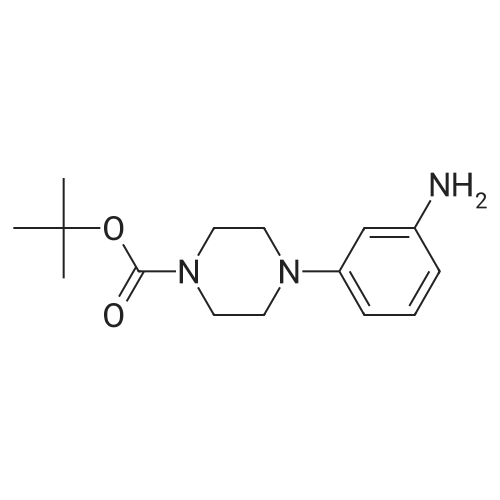
Step 3: tert-butyl 4-(2-aminophenyl)piperazine-1-carboxylate To a solution of tert-butyl-4-(2-nitrophenyl)piperazine-1-carboxylate (1.0 g, 3.25 mmol) in EtOH (30 mL) and H2O (15 mL) at rt was added NH4Cl (870 mg, 16.25 mmol) and Fe (1.09 g, 19.5 mmol). The reaction mixture was stirred at 90 C. for 2 hrs, then cooled to rt and extracted with EtOAc (50 mL*3). The combined organic layers were washed with brine, dried over Na2SO4, filtered, concentrated, and purified by chromatography (silica, EtOAc/PE=1/5) to afford tert-butyl-4-(2-aminophenyl)piperazine-1-carboxylate (800 mg, 2.88 mmol, 89%) as an oil. ESI-MS (EI+, m/z): 278.4 [M+H]+. |
| 74% |
With 5%-palladium/activated carbon; hydrogen; In methanol; under 7500.75 Torr; for 14h; |
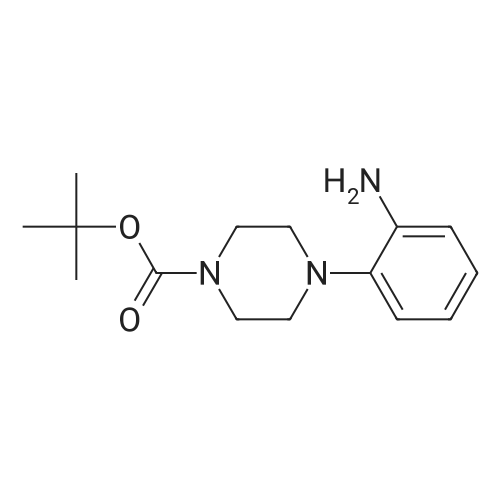
To a solution of lllb (1 .00 g, 3.25 mmol) in MeOH (10 mL) Pd/C (5%, 50 mg) was carefully added. Obtained mixture was hydrogenated under 10 bar H2 for 14 h. Pd/C was then filtered off and solvent evaporated to afford an oil, which crystalized upon standing. The product was recrystallized from MeCy (10 mL), precipitate was filtered off, washed with MeCy (2 x 5 mL) and dried to afford the title compound IVb as light violet powder. (0.67 g, 74% yield): H NMR (CDCI3, 500 MHz) δ 1 .50 (s, 9H), 2.87 (m, 4H), 3.57 (br s, 2H), 3.99 (m, 4H), 6.75 (m, 2H), 6.94-6.99 (m, 2H); MS (ESI) mz. 278 [MH]+. |
| 74% |
With 5%-palladium/activated carbon; hydrogen; In methanol; under 7500.75 Torr; for 14h; |
To a solution of IIIb (1.00 g, 3.25 mmol) in MeOH (10 mL) Pd/C (5%, 50 mg) was carefully added. Obtained mixture was hydrogenated under 10 bar H2 for 14 h. Pd/C was then filtered off and solvent evaporated to afford an oil, which crystalized upon standing. The product was recrystallized from MeCy (10 mL), precipitate was filtered off, washed with MeCy (2 x 5 mL) and dried to afford the title compound IVb as light violet powder. (0.67 g, 74% yield): 1H NMR (CDCl3, 500 MHz) δ 1.50 (s, 9H), 2.87 (m, 4H), 3.57 (br s, 2H), 3.99 (m, 4H), 6.75 (m, 2H), 6.94-6.99 (m, 2H); MS (ESI) m/z: 278 [MH]+. |
|
palladium; In ethanol; |
PREPARATION 13 1-[1,1-Dimethylethoxycarbonyl]-4-(2-aminophenyl)piperazine 1-[1,1-Dimethylethoxycarbonyl]-4-(2-nitrophenyl)piperazine (PREPARATION 12, 7.61 g) is dissolved in ethanol (150 ml). Palladium on carbon (10%, 0.75 g) is added and the reaction is hydrogenated at 45 psi for 6 hr. The mixture is filtered thru celite and concentrated under reduced pressure to give the title compound. |
| 25.3 g |
With 5% Pd/C; hydrogen; In ethanol; at 35 - 40℃; under 1551.49 - 2068.65 Torr; for 10h; |
Ortho-fluoronitrobenzene (14.1 g, 0.1 mol), 4-tert-butoxycarbonyl-1-piperazine (18.6 g, 0.1 mol), and potassium carbonate (13.8 g, 0.4 mol) were added to acetonitrile (140 ml), stirred and heated to reflux. After reacting for 16 h, the reaction system was cooled to room temperature, filtered under reduced pressure to remove inorganic salts. Then, the filter cake was washed with acetonitrile (40 ml), and the filtrate was merged and concentrated to a slurry system under reduced pressure. Ethanol (140 ml) was added and concentrated to obtain a slurry system, after that ethanol (140 ml) was added, and stirred until clarification. Then a wet palladium/carbon (7% palladium) (1.12 g) was added. The system was purged with nitrogen gas (40 psi) for three times and then hydrogen gas (40 psi) for three times. Hydrogenation was carried out, under the pressure of 30 to 40 psi and at the temperature of 35 to 40 C. for 10 h, then cooled to room temperature, and filtered to remove palladium/carbon. The filter cake was washed with ethanol (30 ml), and the filtrate was merged and concentrated to dry under reduced pressure. A pale yellow solid of 25.3 g was obtained, and the yield was 91.2%; MS+=278.2. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping