| 68% |
With tetrakis(triphenylphosphine) palladium(0); potassium carbonate; In tetrahydrofuran; water; at 80℃; for 12h;Inert atmosphere; |
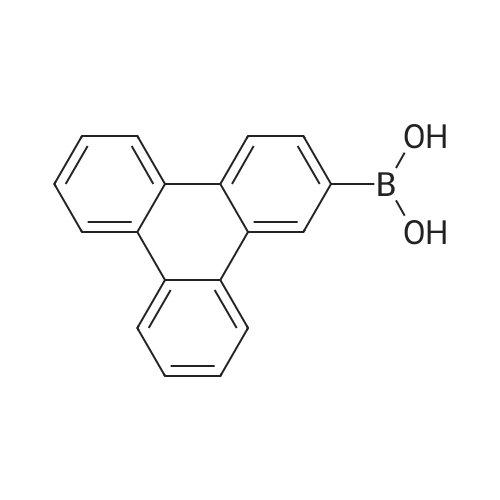
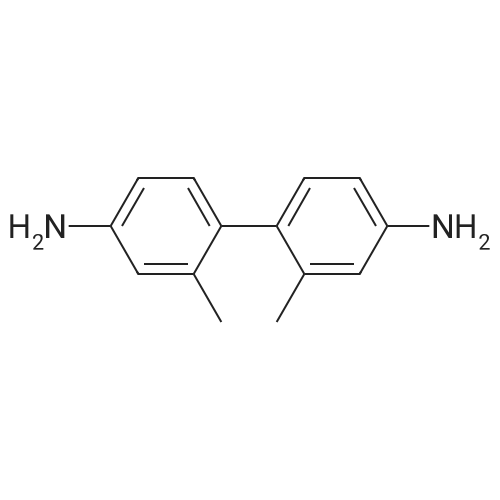
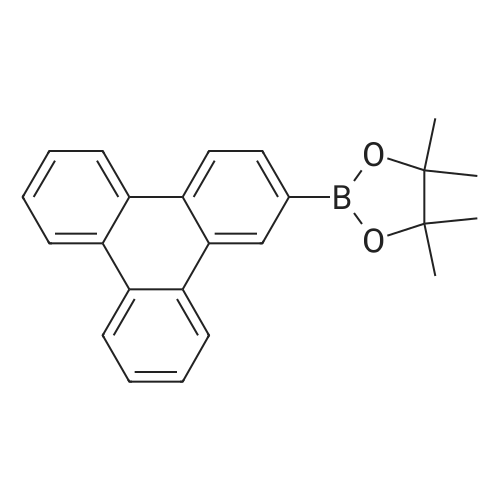
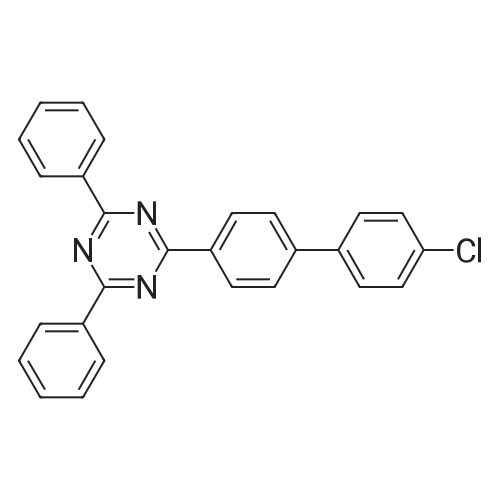
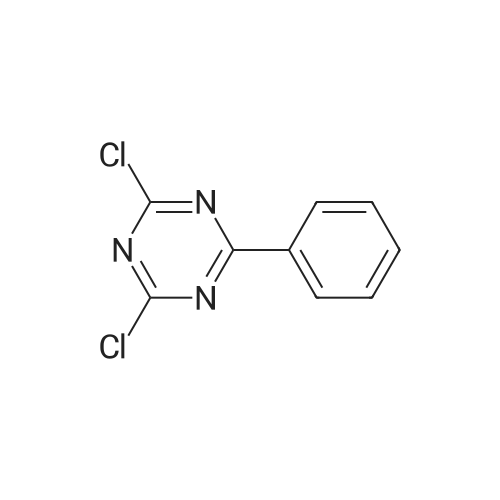
<strong>[890042-13-4]4,4,5,5-tetramethyl-2-(triphenylen-2-yl)-1,3,2-dioxaborolane</strong> (50 g, 141 mmol) was dissolved in 0.5 L of tetrahydrofuran (THF) under a nitrogen environment, and 2,4-dichloro-6-phenyl-1,3,5-triazine (47.9 g, 212 mmol) and tetrakis(triphenylphosphine)palladium (1.63 g, 1.41 mmol) were added thereto and then, stirred. Subsequently, potassium carbonate saturated in water (48.7 g, 353 mmol) was added thereto and then, heated and refluxed at 80 C. for 12 hours. When a reaction was complete, after adding water to the reaction solution, the mixture was extracted with dichloromethane (DCM), treated with anhydrous magnesium sulfate to remove moisture, filtered, and concentrated under a reduced pressure. This obtained residue was separated and purified through flash column chromatography to obtain Intermediate I-1 (40.1 g, 68%). (0147) HRMS (70 eV, EI+): m/z calcd for C27H16ClN3: 417.1033, found: 417. (0148) Elemental Analysis: C, 78%; H, 4% |
| 65% |
With tetrakis(triphenylphosphine) palladium(0); potassium carbonate; In tetrahydrofuran; water; at 80℃; for 8h;Inert atmosphere; |
Will be purchased from P&H Tech Co., Ltd. (http://www.phtech.co.kr/)<strong>[890042-13-4]4,4,5,5-tetramethyl-2-(triphenylene-2-yl)-1,3,2-dioxaborolane</strong> (100g, 282mmol) dissolved in 0.1L Tetrahydrofuran (THF) was added under nitrogen and purchased from Tokyo Chemical Industry Co. Ltd. (http://www.tcichemicals.com/)2,4-dichloro-6-phenyl-1,3,5-triazine (95.7g, 423mmol) and tetrakis(triphenylphosphine)palladium (2.72g, 2.86mmol), then stirred. Subsequently, a saturated aqueous solution of potassium carbonate (97.4 g, 705 mmol) was added thereto, and then heated to reflux at 80C for 8 hours. When the reaction was completed, water was added to the reaction solution, and the mixture was extracted with dichloromethane (DCM), treated with anhydrous magnesium sulfate to remove moisture, filtered, and concentrated under reduced pressure. The resulting residue was separated and purified by flash column chromatography to obtain Intermediate I-1 (76.6 g, 65%). |
| 40.1 g |
With tetrakis(triphenylphosphine) palladium(0); potassium carbonate; In tetrahydrofuran; water; at 80℃; for 12h; |
Dissolve 4,4,5,5-tetramethyl-2-(triphenylene-2-yl)-1,3,2-dioxaborane (50g, 141mmol) in 0.5 of a 1L round bottom flask L tetrahydrofuran (THF), add 2,4-dichloro-6-phenyl-1,3,5-triazine (47.9g, 212mmol) and tetrakis(triphenylphosphine) palladium (1.63g, 1.41 mmol), and the resulting mixture was stirred. Subsequently, a saturated aqueous solution of potassium carbonate (48.7 g, 353 mmol) was added thereto, and the resulting mixture was heated and refluxed at 80C for 12 hours. When the reaction was completed, water was added to the reaction solution, and the resultant was extracted with dichloromethane (DCM), treated with anhydrous magnesium sulfate to remove moisture, filtered, and concentrated under reduced pressure. The residue obtained in this way was separated and purified by flash column chromatography to obtain 40.1 g of intermediate product C-1-4. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping