|
With sodium tetrahydroborate; In methanol; at 0 - 25℃;Inert atmosphere; |
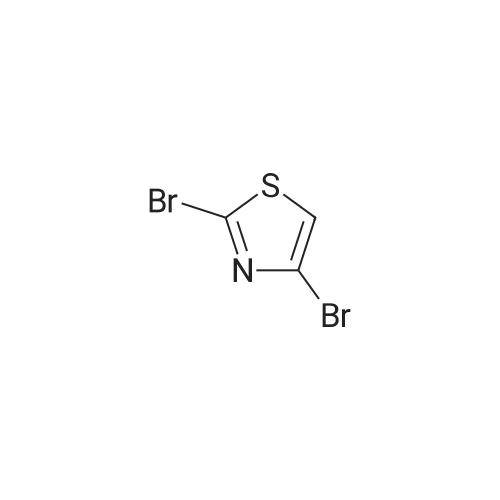
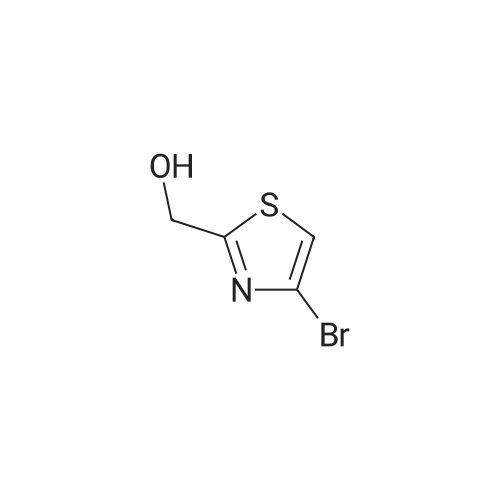
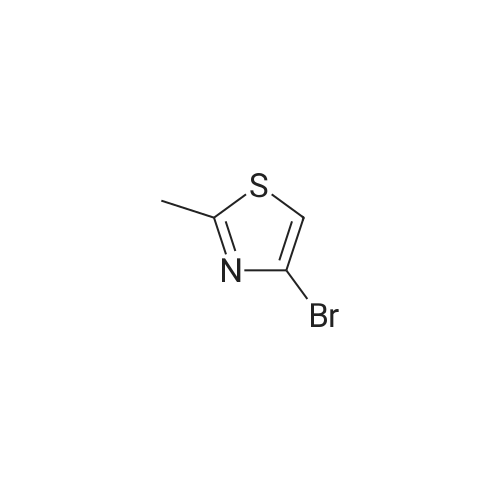
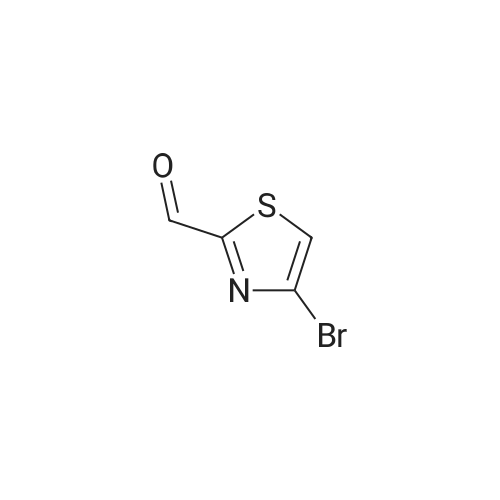
In a flame dried round-bottomed flask equipped with a magnetic stir bar and under inert atmosphere (N2), <strong>[167366-05-4]4-bromo-thiazole-2-carbaldehyde</strong> (1.68 g, 8.75 mmol) was dissolved in MeOH (10 mL). NaBH4 (428 mg, 10.86 mmol) was added portionwise at 0 0C and the reaction mixture stirred at rt for 1 h. Water (10 mL) was added and the mixture extracted with EA (3 x 20 mL). The combined org. extracts were dried over Na2SO4, filtered, and the solvents were removed under reduced pressure. Purification of the residue by FC (6:1 -> 2:1 hept-EA) gave the title compound as a pale yellow solid. TLC: rf (1 :1 hept-EA) = 0.31. LC-MS-conditions 02: tR = 0.62 min [M+H]+ = 194.31. |
|
With sodium tetrahydroborate; In methanol; at 20℃; |
To an ice-cold solution of <strong>[167366-05-4]4-bromothiazole-2-carbaldehyde</strong> (1.78 g, 9.27 mmol, crude obtained above), in methanol (30 ml) was added NaBH4 (1.76 g, 46.35 mmol) portion wise. The resulting reaction mixture was stirred at room temperature for 2 h. After completion of the reaction (TLC monitoring), the reaction mass was cooled to 0 C., quenched it with 25 ml of water and concentrated under vacuum. Added 50 ml water and extracted with EtOAc (3×100 ml). The combined organics was dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. The crude residue was purified over silica gel (60-120 M, 10% EtOAc-Hexane) to get the desired product (1.40 g, 78%). MS: 194.01 (M+H)+. |
|
With sodium tetrahydroborate; In methanol; at 0 - 20℃;Inert atmosphere; |
(4-Bromo-thiazol-2-yl)-methanol:; In a flame dried round-bottomed flask equipped with a magnetic stir bar and under inert atmosphere (N2), <strong>[167366-05-4]4-bromo-thiazole-2-carbaldehyde</strong> (1.68 g, 8.75 mmol) was dissolved in MeOH (10 ml_). NaBH4 (428 mg, 10.86 mmol) was added portionwise at 0 0C and the reaction mixture stirred at rt for 1 h. Water (10 ml.) was added and the mixture extracted with EA (3 x 20 ml_). The combined org. extracts were dried over Na2SO4, filtered, and the solvents were removed under reduced pressure. Purification of the residue by FC (6:1 ->; 2:1 hept-EA) gave the title compound as a pale yellow solid. TLC: rf (1 : 1 hept-EA) = 0.31. LC-MS-conditions 02: tR = 0.62 min [M+H]+ = 194.31. |
|
|
To a solution (20 mL) of crude <strong>[167366-05-4]4-bromo-1,3-thiazole-2-carbaldehyde</strong> in ethanol was added sodium tetrahydroborate (935 mg, 24.7 mmol), and the mixture was stirred at room temperature for 3 hr. Water was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The obtained organic layer was washed with saturated brine, and dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure. The obtained crude product was purified by column chromatography (hexane - hexane/ethyl acetate=75/25) to give the title compound (1.8 g, 44%) as a pale-yellow oil. 1H NMR (300 MHz, CHLOROFORM-d) delta ppm 2.60 (1 H, t, J=6.2 Hz), 4.96 (2 H, d, J=6.2 Hz), 7.22 (1 H, s) |
| 865 mg |
With methanol; sodium tetrahydroborate; In tetrahydrofuran; at 0 - 35℃; for 1h; |
A) (4-bromothiazol-2-yl)methanol To a solution of <strong>[167366-05-4]4-bromothiazole-2-carbaldehyde</strong> (1.20 g) in THF (10 mL) were successively added sodium borohydride (236 mg) and methanol (1.0 mL) at 0C, and the mixture was stirred at room temperature for 1 hr. 1N Hydrochloric acid was added, and the reaction mixture was extracted with ethyl acetate. The extract was washed with saturated brine, and dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure, and the residue was purified by silica gel column chromatography (NH, ethyl acetate/hexane) to give the title compound (865 mg) as a colorless oil. 1H NMR (300 MHz, DMSO-d6) delta 4.72 (2H, d, J = 6.0 Hz), 6.19 (1H, t, J = 5.9 Hz), 7.75 (1H, s). |
| 3.28 g |
With sodium tetrahydroborate; In methanol; at 20℃; for 0.333333h; |
Step 1: (4-Bromo-thiazol-2-yI)-methanol [00308] To a solution of <strong>[167366-05-4]4-bromo-2-formylthiazole</strong> (4.00 g, 20.8 mmol) in methanol (60.0 mL, 1480 mmol) was slowly added sodium tetrahydroborate (0.946 g, 25.0 mmol), and the reaction was stirred at rt for 20 min. The reaction was concentrated in vacuo, diluted with EtOAc, and washed with water 2x and then brine l x. Organic layer was dried over Na2S04, filtered and concentrated in vacuo. The residue was purified via column chromatograpy (40g column, 20% - 50% EtOAc in hexanes over 30min) to give a light yellow oil. Yield = 3.28 g. NMR (400 MHz, Chloroform-d) delta 7.22 (s, 1H), 4.95 (d, J = 6.2 Hz, 2H), 2.76 (t, J = 6.2 Hz, 1H). LCMS (FA): 196.0 m/z (M + 1). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping