|
In chloroform; at 0℃; for 1.16667h;Heating / reflux; |
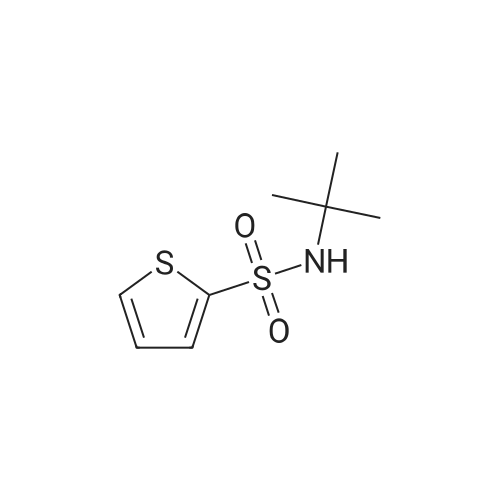
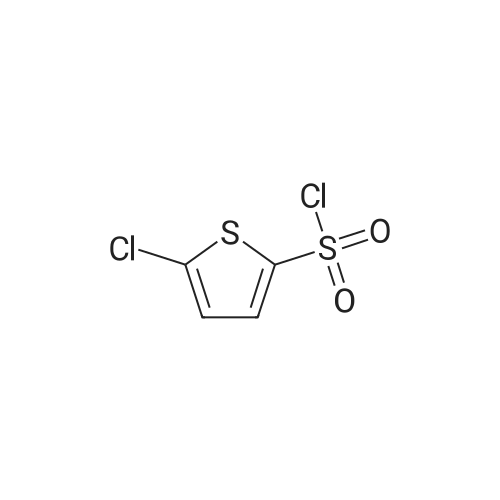
Thiophene-2-sulfonyl chloride (15 g, 0.082 mol) was dissolved in CHC13 (200 mL) under N2 atmosphere and then cooled to 0C. TERT-BUTYLAMINE (25.9 mL, 0.246 mol) dissolved in CHC13 (50 ML) was then added dropwise to the reaction mixture. The reaction mixture was stirred for 1 hour at room temperature and then at reflux for 10 min. Toluene (700 mL) was added and the organic phase was washed with water (3 x 50 mL), dried, and concentrated in vacuo. The sub-title product was used without further purification in the next step. 1H NMR No.(CDCL3) : 7.60 (1H, dd, J= 1.3, 3.8 Hz), 7.53 (1H, dd, J= 1.3, 5.0 Hz), 7.02 (1H, dd, J = 5.0, 3.8 Hz), 5. 13- (1 H, m), 1.24 (9H, m) 13C NMR 6 (CDC13) : 145.0, 131.7, 131.2, 127.0, 55.1, 29.9 |
|
In chloroform; at 20℃; for 1.16667h;Heating / reflux; |
Thiophene-2-sulfonyl chloride (15 g, 0.082 mol) was dissolved in CHC13 (200 mL) under N2 atmosphere and then cooled to 0C. tert-Butylamine (25.9 mL, 0.246 mol) dissolved in CHC13 (50 ML) was then added dropwise to the reaction mixture. The reaction mixture was stirred for 1 h at room temperature and then at reflux for 10 min. Toluene (700 mL) was added and the organic phase was washed with water (3 x 50 mL), dried, and concentrated IFS vacuo. The sub-title product was used without further purification in the next step. LH NMR B (CDC13) : 7.60 (1H, dd, J= 1.3, 3.8 Hz), 7.53 (1H, dd, J= 1.3, 5.0 Hz), 7.02 (1H, dd, J = 5. 0,3. 8 HZ), 5.13 (1H, m), 1.24 (9H, m) 13C NMR 8 (CDC13) : 145.0, 131.7, 131.2, 127.0, 55.1, 29.9 |
|
In chloroform; at 0 - 20℃; for 1.16667h;Heating / reflux; |
Thiophene-2-sulfonyl chloride (15 g, 0.082 mol) was dissolved in CHC13 (200 mL) under N2 atmosphere and then cooled to 0oC. tert-Butylamine (25.9 ML9 0.246 mol) dissolved in CHC13 (50 mL) was then added dropwise to the reaction mixture. The reaction mixture was stirred for 1 h at room temperature and then at reflux for 10 min. Toluene (700 mL) was added and the organic phase was washed with water (3 x 50 mL), dried, and concentrated in vacuo. The sub-title product was used without further purification in the next step. 'H NMR (CDC13) 8 7.60 (1H, dd, J=1.3, 3.8 Hz), 7.53 (1H, dd, J=1.3, 5.0 Hz), 7.02 (1H, dd, J=5.0, 3. 8 Hz), 5.13 (1H, m), 1.24 (9H, M) 13C NMR (CDC13) 5 145.0, 131.7, 131.2, 127.0, 55.1, 29.9 |
|
In chloroform; at 0 - 20℃; for 1.16667h;Heating / reflux; |
(a) LambdaLfe7Y-Butylthiophene-2-sulfonamide; Thiophene-2-sulfonyl chloride (15 g, 0.082 mol) was dissolved in CHCl3 (200 mL) under N2 atmosphere and then cooled to 00C. tert-Butylamine (25.9 mL, 0.246 mol) dissolved in CHCl3 (50 mL) was then added' drop wise to the reaction mixture. The reaction mixture was stirred for 1 h at room temperature and then at reflux for 10 min. Toluene (700 mL) was added and the organic phase was washed with water (3 x 50 mL), dried, and concentrated in vacuo. The sub-title product was used without further purification in the next step.1H NMR delta (CDCl3): 7.60 (IH, dd, J=1.3, 3.8 Hz), 7.53 (IH, dd5 J=I .3, 5.0 Hz), 7.02 (IH, dd, J=5.0, 3.8 Hz), 5.13(1H, m), 1.24 (9H5 m). 13C NMR delta (CDCl3): 145.0, 131.7, 131.2, 127.0, 55.1, 29.9. |
|
In chloroform; at 0 - 20℃; for 1.16667h;Heating / reflux; |
(a) N-fe7f-Butylthiopliene-2-sulfonamide;Thiophene-2-sulfonyl chloride (15 g, 0.082 mol) was dissolved in CHCl3 (200 mL) under N2 atmosphere and then cooled to 0C. fez-f-Butylamine (25.9 mL, 0.246 mol) dissolved in CHCl3 (50 mL) was then added dropwise to the reaction mixture. The reaction mixture was stirred for 1 h at room temperature and then at reflux for 10 min. Toluene (700 mL) was added and the organic phase was washed with water (3 x 50 mL), dried, and concentrated in vacuo. The sub-title product was used without further purification in the next step. 1H NMR (CDCl3) delta 7.60 (IH, dd, J=1.3, 3.8 Hz), 7.53 (IH, dd, J=1.3, 5.0 Hz), 7.02 (IH, dd, J=5.0, 3.8 Hz), 5.13 (IH, m), 1.24 (9H, m). 13C NMR (CDCl3) delta 145.0, 131.7, 131.2, 127.0, 55.1, 29.9. |
|
In chloroform; at 0 - 20℃; for 1.16667h;Heating / reflux; |
(a) iV-fert-Butylthiophene-2-sulfonamide; Thiophene-2-sulfonyl chloride (15 g, 0.082 mol) was dissolved in CHCl3 (200 mL) under N2 atmosphere and then cooled to 0C. fe/Y-Butylamine (25.9 mL, 0.246 mol) dissolved in CHCl3 (50 mL) was then added dropwise to the reaction mixture. The reaction mixture was stirred for 1 h at room temperature and then at reflux for 10 min. Toluene (700 mL) was added and the organic phase was washed with water (3 x 50 mL), dried, and concentrated in vacuo. The sub-title product was used without further purification in the next step.1H NMR delta (CDCl3): 7.60 (IH, dd, J=I .3, 3.8 Hz)5 7.53 (IH, dd, J=1.3, 5.0 Hz), 7.02 (IH5 dd, J=5.0, 3.8 Hz)5 5.13(1H, m), 1.24 (9H5 m). 13C NMR delta (CDCl3): 145.0, 131.7, 131.2, 127.0, 55.1, 29.9. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping