| 97% |
With tin(II) chloride dihdyrate; In ethanol; for 3.0h;Reflux; |
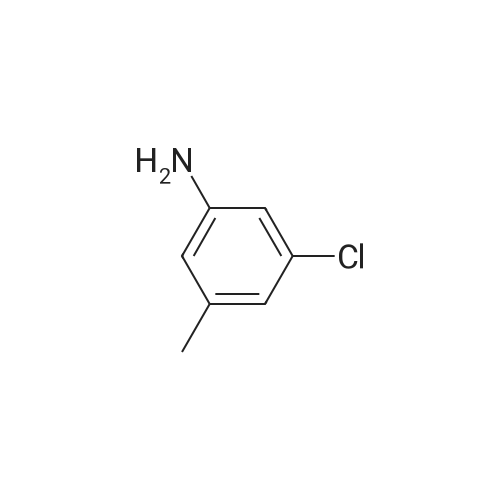
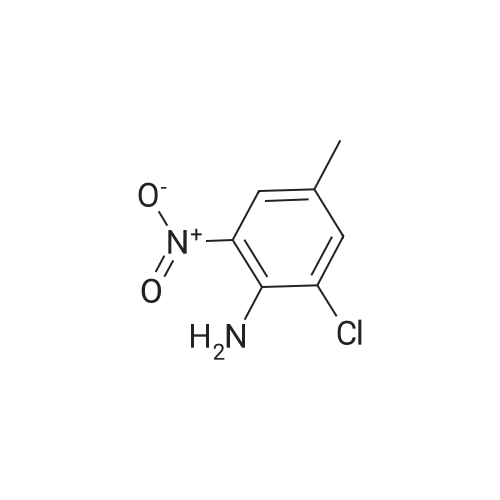
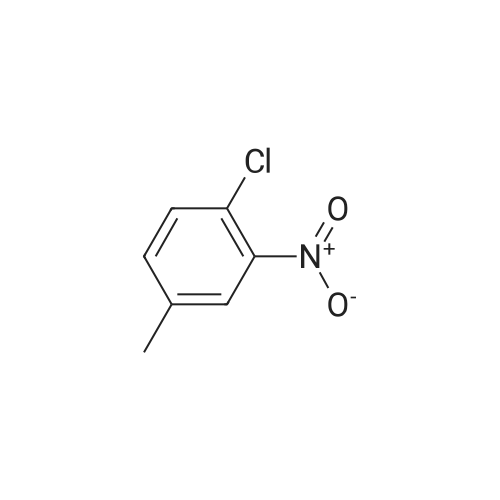
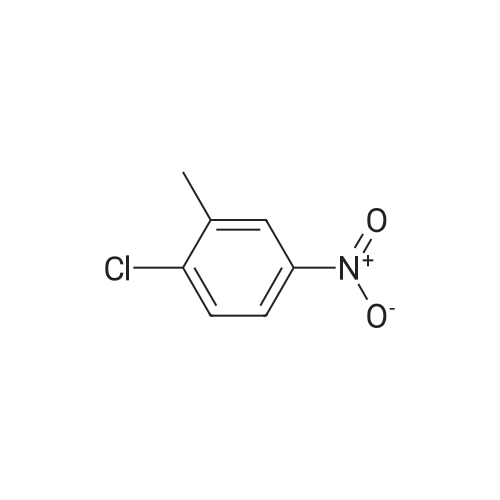
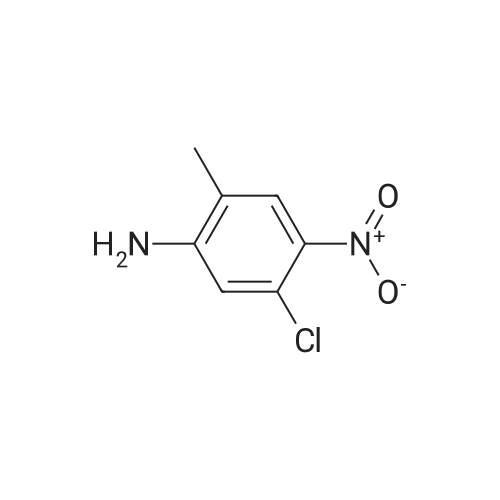
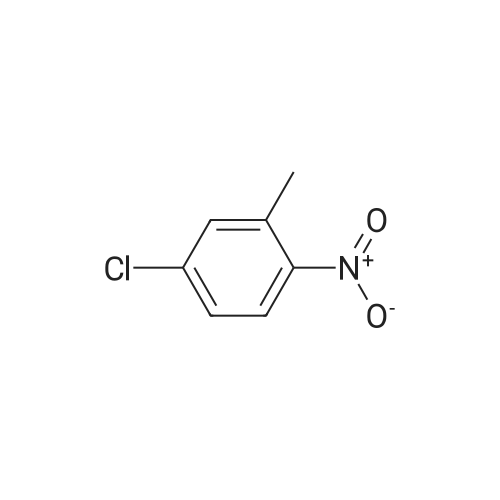
3-Chloro-5-methyl-phenylamine. An ethanol solution (75 mL) containing <strong>[16582-38-0]1-chloro-3-methyl-5-nitro-benzene</strong> (5.0 g, 29 mmol) are added with SnCl2.2H2O (32.8 g, 146 mmol). The reaction mixture was reflux for 3.0 h. The solution was concentrated under vacuum, and the residue was re-dissolved in aqueous NaOH, filtered, and extracted with EtOAc. The organic layer was collected, washed with brine, dried over MgSO4, and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel to give 3-chloro-5-methyl-phenylamine (4.0 g) as light yellow solids in 97% yield: 1H NMR (500 MHz, CDCl3) delta 6.56 (s, 1H), 6.48 (s, 1H), 6.36 (s, 1H), 3.66 (bs, 2H), 2.23 (s, 3H); ESI-MS: m/z 141.7 (M+H)+. |
| 97% |
With tin(II) chloride dihdyrate; ethanol; for 3.0h;Reflux; |
An ethanol solution (75 mL) containing <strong>[16582-38-0]1-chloro-3-methyl-5-nitro-benzene</strong> (5.0 g, 29 mmol) were mixed with SnCl2.2H2O (32.8 g, 146 mmol). The reaction mixture was refluxed for 3.0 h. The solution was concentrated under vacuum, and the residue was re-dissolved in aqueous NaOH, filtered, and extracted with EtOA.c. The organic layer was collected, washed with brine, dried over MgSO4(s), and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel to give 3-chloro-5- methyl-phenylamine (4.0 g) as light yellow solids in 97% yield: 1H NMR (500 MHz, CDCl3) delta 6.56 (s, 1 H), 6.48 (s, 1 H), 6.36 (s, 1 ), 3.66 (s, 2 H), 2.23 (s, 3 H); ESI-MS: m/z 141.7 (M + H)+. |
| 80% |
|
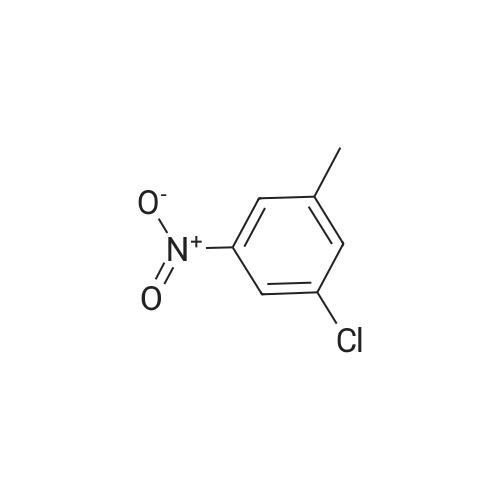
3-Chloro-5-nitrotoluene (45 g, 0.241 mol) and ethanol (500 ml) were mixed in a 2 litter four-neck flask. The mixture was cooled to about 4 ° C. A solution of tin chloride monohydrate (217.67 g, 0.965 mol) in 200 ml of ethanol was added dropwise to the mixture over 2 hours, while the reaction mixture was maintained at 10 ° C. or lower. Then the reaction product mixture was stirred at room temperature for 2 hours and poured into 2500 ml of iced water. It was neutralized with sodium hydroxide and filtered with a nutsche filled with sellaite. The residue was washed with ethyl acetate. The intended product was obtained with ethyl acetate from the filtrate liquid. Then the extract liquid and the washing liquid were jointed. The mixture was washed with water and then a saturated salt water and dried with magnesium sulfate. Concentrated, it was treated by distillation at a reduced pressure to obtain 28.0 g of a yellow liquid (production yield 80%). B.p.: 85-92 ° C./0.4 kPa or lower (3 Torr or lower) 1H-NMR, 500 MHz, in CDCl3 (delta) 6.56 (bs, 1H), 6.48 (dd, JI, J2=1.3 Hz, 1H), 6.36 (bs, 1H), 3.64 (bs, 2H), 2.22 (s, 3H) |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping