| 92.7% |
With potassium hydroxide; potassium iodide In dimethyl sulfoxide at 0 - 20℃; for 4 h; |
EXAMPLE 1
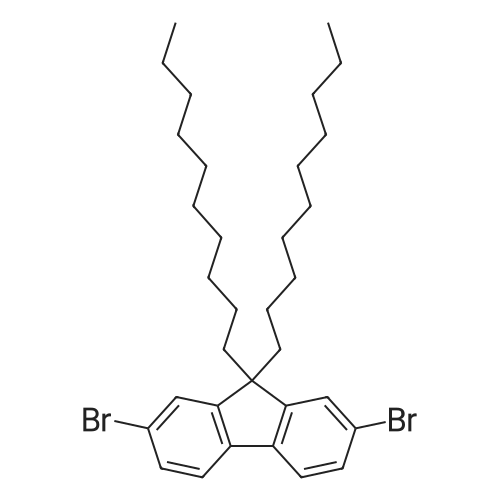
2,7-Dibromo-9,9-didecyifluorene
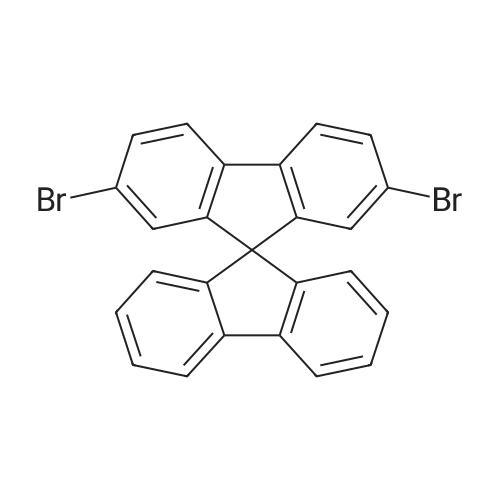
A mixture of 2,7-dibromofluorene (64.8 g, 0.20 mol potassium iodide (3.0 g, 0.018 mol), finely powdered KOH (56.0 g, 1 mol), and DMSO (150 ML) was mechanically stirred under nitrogen in a three-necked round-bottom flask..
The flask was cooled in an ice water bath before adding four batches of the bromoalkyl (90 ML. 95.94 g, 0.434 mol)..
After each addition, the internal temperature rose sharply..
Once the last aliquot was completed, the reaction was stirred for 4 hours at room temperature before water (200 ML) was added..
The solid was filtered off and dried in a desiccator equipped with P2O5 under vacuum for 16 hours..
The crude product was recrystallized from ethanol to produce 2,7-dibromo-9,9-didecylfluorene as white crystals (m.p. 40-41° C.) in 92.7percent yield..
Mass Spec. m/z 602,604,606 (M+), 461,463,465 (M-C10H21), 382,384 (461-Br). Anal. Calcd for C33H48Br2: C, 65.56percent; H, 8.00percent; Br, 26.44percent. Found: C, 64.99percent; H, 8.21percent; Br, 27.25percent.
|
| 92% |
With tetrabutylammomium bromide; sodium hydroxide In water; dimethyl sulfoxide at 20℃; for 4 h; |
2.2.1
2,7-dibromo-9,9-didecylfluorene (1)
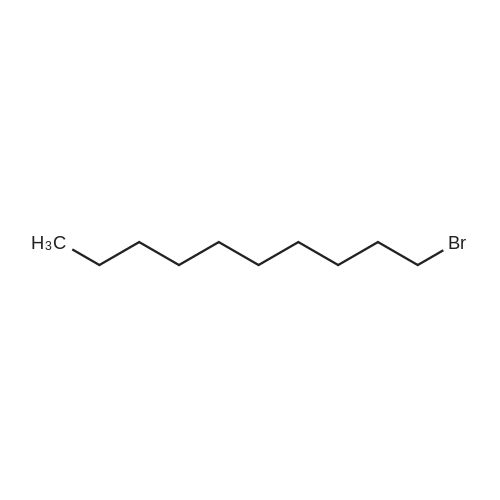
1-bromodecane (4.42 g, 20.0 mmol) was added by syringe to a mixture of 2,7-dibromofluorene (2.59 g, 8 mmol), tetrabutyl ammonium bromide (0.02 g, 0.064 mmol) and 2.50 mL of 50percent aqueous sodium hydroxide in dimethyl sulfoxide (50.0 mL).
After stirring at room temperature for 4 h, the mixture was poured into water and extracted three times with dichloromethane.
The solvent was removed under reduced pressure and the precipitate was purified by column chromatography on silica gel (eluent: petroleum ether) to afford 2,7-dibromo-9,9-didecylfluorene as a white powder (4.45 g, Yield 92percent).
1H NMR (500 MHz, CDCl3, δ): 7.51 (d, 2H, J = 8.70 Hz, Ar-H), 7.45 (m, 4H, Ar-H), 1.91 (m, 4H, CH2), 1.13 (m, 28H, CH2), 0.85 (t, 6H, J = 6.95 Hz, CH3), 0.58 (m, 4H, CH2). FT-IR (cm-1, KBr): ν = 2950, 2836 (s; CH), 1638, 1551 (w; Ar). Anal. Calcd. for C33H48Br2: C 65.56, H 8.00; Found: C 64.72, H 7.62. |
| 90.7% |
Stage #1: With potassium <i>tert</i>-butylate In tetrahydrofuranInert atmosphere
Stage #2: for 5.5 h; Cooling with liquid nitrogen |
To a 2 L three-necked round bottom flask equipped with mechanical stir, nitrogen inlet and outlet, 81.00 g (0.250 mol) 25 2,7-dibromo-9H-fluorence and 500 mL 11 THF were charged. After the 2,7-dibromofluorence was dissolved, 58.9 g (0.525 mol) 26 potassium t-butoxide was added in three batch. The mixture was turned from colorless to dark red immediately, 116.1 g (0.525 mol) of 27 1-bromodecane in 150 mL THF was added drop wise within 3.5 hrs. After addition completed, the mixture was stirred under nitrogen for 2 hrs. Potassium salts was removed through filtration. The filtrate was concentrated under vacuum to give yellow viscous oil. The final product was purified by silica gel chromatography using hexanes as eluent. 137.1 g 28 product was obtained as waxy crystals, 90.7percent isolated yield, m.p. 37.8-39.0° C. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping