| 97% |
With triethylamine; In dichloromethane; at 0 - 20℃; |
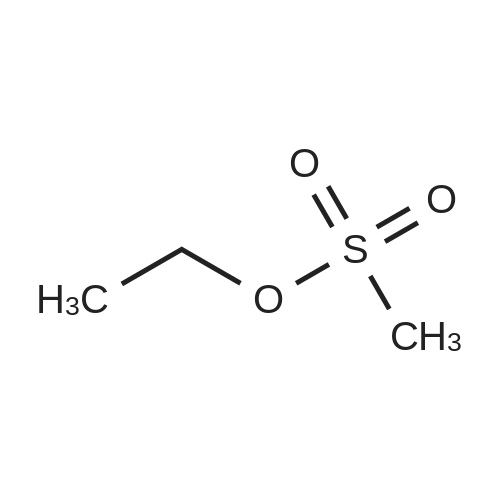
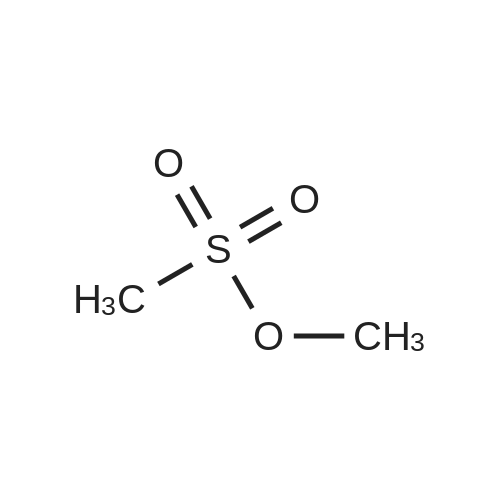
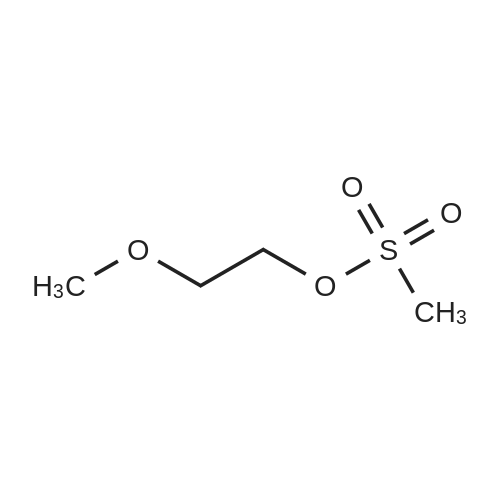
7.6 g (0.1 mol) of ethylene glycol monomethyl ether 24-1 was dissolved in 150 mL of anhydrous dichloromethane, 20 g of triethylamine was added, 0.1 M of a solution of methanesulfonyl chloride (0.11 mol) in dichloromethane was added dropwise at 0 C. After the addition was complete, the mixture was reacted at room temperature overnight, washed with water, dried and concentrated to obtain 14.9 g of ethylene glycol monomethyl ether protected with Ms 24-2, yield 97%. |
| 96% |
With triethylamine; In dichloromethane; for 1h;Cooling with ice; |
An ice-cold solution of compound 79 (5 g, 65.7 mmol) and triethylamine (11 ml, 79 mmol) in DCM (150 mL) was treated with methanesulfonyl chloride (5.59 ml, 72.3 mmol) over 2 min. After 1 h the reaction was judged to be complete by TLC. Water was added and the organic extracts were washed sequentially with dilute HC1, saturated sodium bicarbonate, brine, then dried (MgS04), filtered and reduced. The product mesylate (9.4 g, 61 mmol, 96%>) was taken up in acetone (200 ml), then the potassium salt of toluene thiosulfmic acid (61 mmol) was added and the solution was stirred at 50C over 18 h. A thick white precipitate was observed to form. The mixture was filtered, reduced to an oil then extracted into DCM/water. The organicextracts were dried (MgS04), filtered and reduced. Column chromatography gave pure compound 80 as a colorless oil which crystallized on standing Rf = 0.3 (20% EA/hexane) |
| 92% |
In dichloromethane; at 20℃; for 2h; |
2-methoxyethanol (1, 6 ml, 0.075 mol) was dissolved in CH2Cl2 (102 ml). The solution was cooled on an ice bath (5 min) and then trimethylamine (20.7 ml, 0.15 mol) was added. Afterward, a solution of mesyl chloride (9.3 ml, 0.075 mol) in CH2Cl2 (18 ml) was added dropwise. The solution was kept at room temperature for 2 h. After this, the crude was poured onto a mixture of water/ice which contains HCl (20 ml). Then, the organic phase was decanted and the aqueous suspension extracted with CH2Cl2 (3 x 30 mL). Finally, the combined organic phases were washed with water (3 x 30 mL), dried with anhydrous MgSO4 and the solvent evaporated in a rotary evaporator. Product 2 was obtained as a yellow oil (92%, 0.069 mmol). |
| 91% |
With triethylamine; In dichloromethane; for 2.5h; |
2-Methoxyethanol (1a, 16 mL, 0.2 mol) was dissolved in dichloromethane (270 mL) in a 2 Lround-bottomed flask. The solution was kept in an ice bath for 15 min, and then triethylamine(55.2 mL, 0.4 mol) was added to the solution. Afterwards, a solution of methanesulfonyl chloride indichloromethane (0.32 mol in 48 mL) was added dropwise for 60 min to the initial reaction crude.After this addition, reaction was stirred at room temperature for another 90 min period. Then, thecrude was poured onto a water/ice mixture containing concentrated hydrochloric acid (50 mL), andthe organic layer was separated, washed three times with brine and dried with anhydrous MgSO4.Dichloromethane was eliminated in a rotary evaporator to give 1b as yellow oil (25.16 g, 0.18 mol, 91%yield). Spectroscopic data were coincident with those reported in the literature. |
| 65% |
With trifluoroacetic acid; In dichloromethane; at 20℃; |
Methanesulfonyl chloride (15 g, 0.13 mol) was added to a mixture of 2-methoxy ethanol (10 g, 0.13 mol), triethylamine (26.5 g, 0.26 mol) and dichloromethane (150 g) at 0 C. After being stirred at room temperature overnight, water (100 g) was added to the reaction mixture. The layers were separated and the organic layer was concentrated in vacuo at room temperature. This gave 13.1 g (65%) of the title mesylate as an oil. 1H NMR analysis supports the stated structure. *Previously reported in Tetrahedron 1995,51, 4867-4890. |
|
In dichloromethane; at 25℃; for 3h; |
Methanesulfonyl chloride (250 g), ethylene glycol monomethyl ether (97.8 g) and methylene chloride (587 mL) were added and the temperature was reduced to 0-5 C.(144 g) was slowly added and the reaction was allowed to proceed at 25 C for 3 hours. TLC indicated that the reaction was completed and 1360 g of ice water was added,And the layers were separated. The aqueous layer was extracted with dichloromethane (645 mL X2), the methylene chloride layers were combined, washed with 635 g of water,20.5 glmol / L hydrochloric acid (pH |
|
With triethylamine; In dichloromethane; at 10℃; for 1.5h;Cooling with ice; |
B1,190 mL of solvent dichloromethane,40 mL of ethylene glycol methyl ether and 210 mL of triethylamine (TEA) were added to a 1000 mL four-necked flask in that order.39 mL of methanesulfonyl chloride and 40 mL of methylene chloride (dilution) were added to a 250 mL constant pressure dropping funnel;The four-necked flask was placed in an ice-water bath,The dropping reaction was started,Adjust the drip rate of 1.5ml / min,The reaction temperature was controlled to 10 C or lower,After completion of the dropwise addition,Stirring 1.5h stop,Drop process system appears white mist,Four-mouth flask with yellow-white solid attached;B2, comprising the steps of:evaporation:The reactants obtained in step b1 were subjected to rotary evaporation under reduced pressure using a reaction apparatus consisting of a circulating vacuum pump and a rotary evaporator,The temperature of the rotary evaporator was set at 50 C,Solvent dichloromethane and acid-binding agent triethylamine gradually steamed out,To be no dripping,Continue to rotate evaporation 20min washing:The evaporated material was dissolved in about 100 ml of dichloromethane,Followed by suction filtration,The filtrate contains the reaction product,The filtrate was collected,Discard the cake triethylamine salt;The filtrate was pickled 5 times,Using a mixed solution of hydrochloric acid and water in a volume ratio of 1: (3 to 5)The remaining triethylamine in the filtrate was removed,The upper layer of the separatory funnel was a hydrochloric acid solution in which triethylamine salt was dissolved,Abandoned,The lower layer is a methylene chloride solution in which the reaction product is dissolved,collect;The methylene chloride solution was washed with water 5 times,The residual hydrochloric acid was removed,Until the water layer close to neutral,The upper layer of the separatory funnel was an aqueous layer containing hydrochloric acid,Abandoned,The lower layer is a methylene chloride solution which dissolves the reaction product,collect;dry:To the dichloromethane solution was added anhydrous MgSO4 overnight,The MgSO4 was filtered off,Then subjected to rotary evaporation,Methylene chloride was distilled off;Vacuum distillation:The material left by the rotary evaporation was distilled under reduced pressure,The distillate was collected at 98 & lt; 0 &That is, ethylene glycol methyl ether methyl sulfonate,Its structure is as follows:Sealed into the dryer to save reserve; |
|
With triethylamine; In toluene; at 20℃; for 0.5h;Cooling with ice; |
Dissolve 5g of 2-methoxyethanol in 10ml of toluene.Add 5.6 ml of triethylamine,5.6 ml of methanesulfonyl chloride was added slowly under ice bath.A large amount of white solid precipitated during the addition.After the addition, the ice bath was removed.Stir at room temperature for 30 min.TLC showed that the reaction of raw materials was completed.After the reaction solution is evaporated,Add 30ml of ethyl acetate and 30ml of water,Divide the water layer,After the organic layer is dry,concentrate,The crude 2-methoxy ethoxy carboxylic acid ester was obtained.Take 200mg crude product dissolved in 10ml DMF,230 mg of 4-chloro-7-hydroxyquinoline was added in succession.358mg cesium carbonate,After putting it in a 50C oil bath for 3 hours,TLC showed that the reaction of the raw materials was completed.After stopping the reaction,After the reaction liquid cools,The DMF is distilled off under reduced pressure.To the residue was added 30 ml of ethyl acetate and 30 ml of water.Extraction and separation of water layer,The organic layer is washed 3 times.30ml each time.After the organic layer is dry,concentrate,The crude 4-chloro-7-methoxyethoxyquinoline (off-white solid, quantitative yield) was obtained. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping