| 40.5% |
With thionyl chloride; In water; N,N-dimethyl-formamide; acetonitrile; |
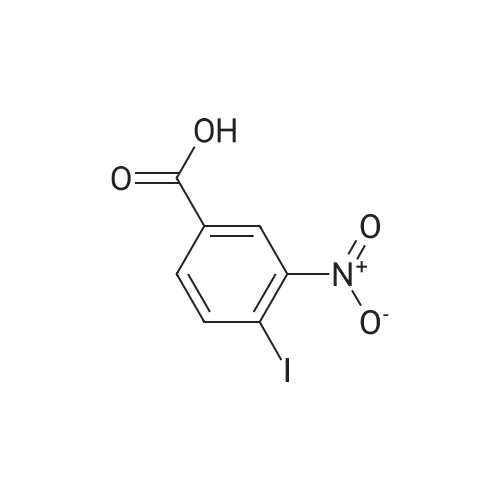
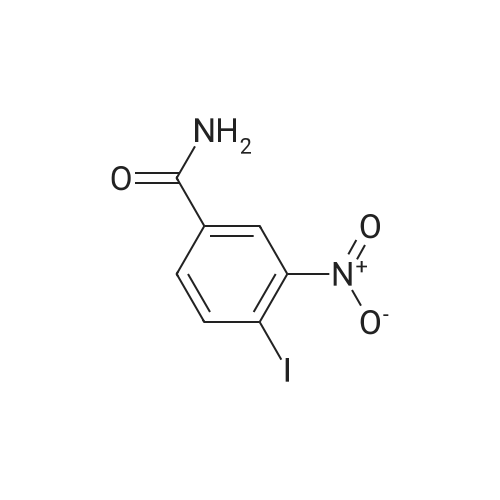
C. 4-Iodo-3-Nitrobenzamide In a 100-mL flask equipped with a magnetic stirrer, thermometer and ice bath, a stirred solution of <strong>[35674-27-2]4-Iodo-3-nitrobenzoic acid</strong> (1025 mg, 3.50 mMoles) (Chemica Alta Ltd., Edmonton, Alberta, Canada) in N,N-dimethylformamide (10 mL) is cooled to 10 C., and then thionyl chloride (0.76 mL, 10.5 mMoles) is added to it. There is no exothermicity, the ice bath is removed, and the solution is allowed to warm to ambient temperature, and stirring is continued for a total of 1 hour. Then the solution is poured into chilled, concentrated ammonium hydroxide (20 mL), resulting in a dark yellow mixture, which is stirred for 5 minutes. Then chilled deionized water (50 mL) is added, causing precipitation of the light yellow product. After allowing the precipitation mixture to stand chilled on ice for 10 minutes, the precipitate is collected on a suction filter, rinsed with cold water, and then dried by vacuum pumping. The resultant crude product (500.4 mg) is then re-crystallized by dissolving it in acetonitrile (7.0 mL) heated to about 65 C., followed by cooling and allowing the solution to stand in the refrigerator overnight. The yellow crystals are collected, rinsed with chilled solvent and dried by vacuum pumping, to give 415.2 mg (40.5% yield) of 4-Iodo-3-nitrobenzamide, m.p. 152-155 C. 1 H NMR spectrum, in DMSO-d6 delta (ppm) values relative to TMS): broad singlet (7.67) due to one nonequivalent proton of the amido NH2 group; doublet of doublets (7.84, 7.85 and 7.86, 7.87) due to H-5 split by H-6 and finely split by H-2; doublet (8.22, 8.24) due to H-6 split by H-5; broad singlet centered near 8.22, overlapping the signal of H-6, due to the second nonequivalent proton of the amido NH2 group; doublet (8.35, 8.36) due to H-2 finely split by H-5. At higher NMR field signals due to adventitious water (2.5 ppm), deuterated-DMSO impurity protons (3.3 ppm) and crystallization solvent acetonitrile (single at 2.07 ppm) are observed. Integration of the acetonitrile signal indicates approximately one molecule of acetonitrile per 3 molecules of 4-iodo-3-nitrobenzamide. UV absorption spectrum in absolute ethanol, lambda max (epsilon): 308 nm (1.59*103), 242 nm (1.31*104), 208 nm (1.45*104) Elemental analysis (Schwarzkopf Microanalytical Laboratory): Calculated for C7 H5 N2 O3 I: C, 28.79%; H, 1.72; I, 43.46; N, 9.59. Found: C, 29.63; H, 1.72; I, 41.47; N, 9.99. Deviations from calculated are believed to be due to the presence of acetonitrile (crystallization solvent) as detected in the NMR spectrum. High resolution EI mass spectrum: calculated for C7 H5 N2 O3 I: 291.9345; Found: M+ (m/z) 291.9349 (deviation=-1.4 ppm). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping