|
|
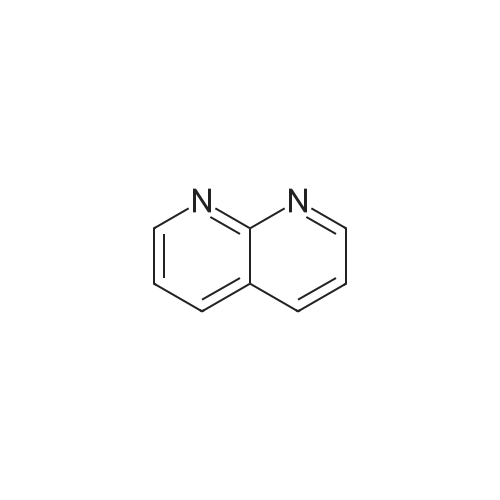
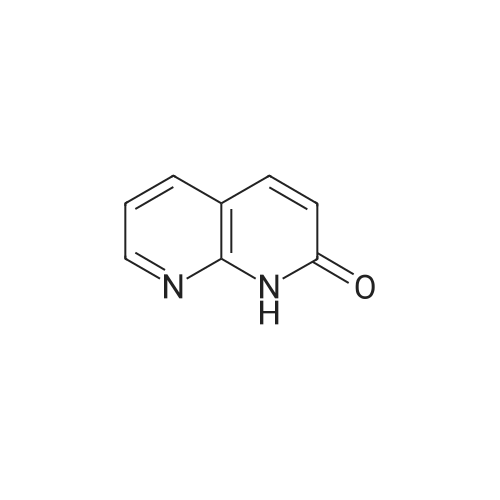
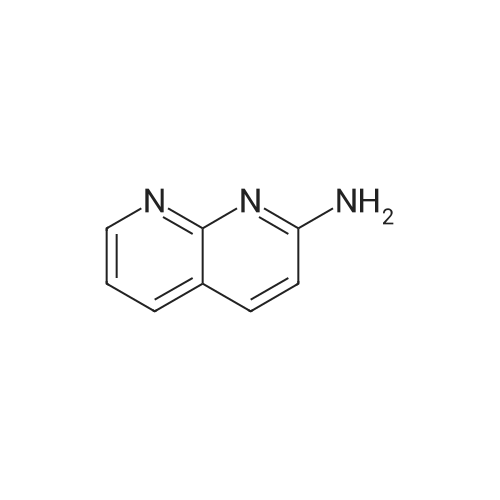
EXAMPLE 16 5-Hydroxy-6-(1,8-naphthyridin-2-yl)-7-oxo-2,3,6,7-tetrahydro-1,4-dithiino[2,3-c]pyrrole (8.45 g.) is added, at 0 C, to a suspension of sodium hydride (0.8 g.) in anhydrous dimethylformamide (125 cc.). The reaction mixture is stirred for half an hour at 0 C, and 1-chlorocarbonyl-4-methylpiperazine (8.45 g.) dissolved in anhydrous dimethylformamide (50 cc.) is then added. After stirring for 21/2 hours at 5 C., the reaction mixture is diluted with ice-water (500 cc.). The precipitate which has appeared is filtered off, washed four times with distilled water (total 200 cc.) and twice with diethyl ether (total 50 cc.) and then dissolved in methylene chloride (300 cc.). The organic solution is washed four times with distilled water (total 160 cc.), dried over anhydrous sodium sulphate, treated with decolourising charcoal (0.2 g.) and evaporated. When the residue liquid reaches a volume of 50 cc., boiling acetonitrile (200 cc.) is added. After cooling for 2 hours at 2 C., the crystals which have appeared are filtered off, washed three times with ice-cold acetonitrile (total 15 cc.) and dried under reduced pressure (20 mm. Hg). The crystals thus isolated (8.0 g.) are dissolved in boiling acetonitrile (270 cc.), decolourising charcoal (0.2 g.) is added to the boiling solution and the whole is filtered. After 24 hours at 2 C., the crystals which have appeared are filtered off, washed three times with ice-cold acetonitrile (total 10 cc.) and dried under reduced pressure (20 mm. Hg). 5-(4-Methylpiperazin-1-yl)carbonyloxy-6-(1,8-naphthyridin-2-yl)-7-oxo-2,3,6,7-tetrahydro-1,4-dithiino[2,3-c]pyrrole (5.8 g.), melting at 255 C., is thus obtained. 5-Hydroxy-6-(1,8-naphthyridin-2-yl)-7-oxo-2,3,6,7-tetrahydro-1,4-dithiino[2,3-c]pyrrole employed as starting material can be obtained in the following way: Preparation of 2-amino-1,8-naphthyridine, m.p. 142 C., according to W. W. |
|
|
EXAMPLE 11 4-Methylpiperazine (8 g.) is added all at once to a suspension of 2-(1,8-naphthyridin-2-yl)-3-phenoxy-carbonyloxy-isoindolin-1-one (5.6 g.) in acetonitrile (100 cc.). The solution obtained is stirred for 6 hours at a temperature of about 20 C. The reaction mixture is poured into a suspension of ice (100 g.) in methylene chloride (300 cc.). An 8% aqueous solution of sodium bicarbonate (200 cc.) is added to the suspension obtained. The organic phase is decanted and the aqueous phase is extracted with methylene chloride (400 cc.). The combined organic phases are dried over anhydrous potassium carbonate (10 g.) and concentrated to dryness. The oily residue (8 g.) is taken up in refluxing diisopropyl ether (100 cc.). On cooling the solution, crystals are deposited and are filtered off. 3-(4-Methylpiperazin-1-yl)carbonyloxy-2-(1,8-naphthyridin-2-yl)isoindolin-1-one (2.9 g.), which melts at 183 C., is thus obtained. 2-(1,8-Naphthyridin-2-yl)-3-phenoxycarbonyloxy-isoindolin-1-one can be prepared in the following way: Preparation of 2-amino-1,8-naphthyridine, m.p. 141 C., according to W. W. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping