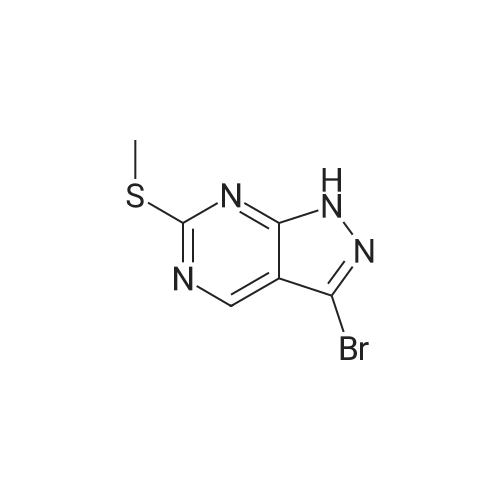
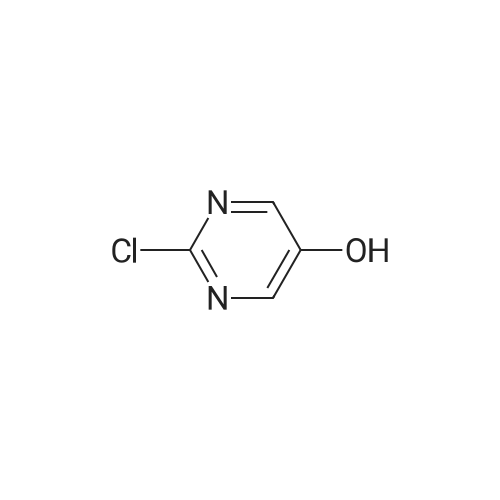
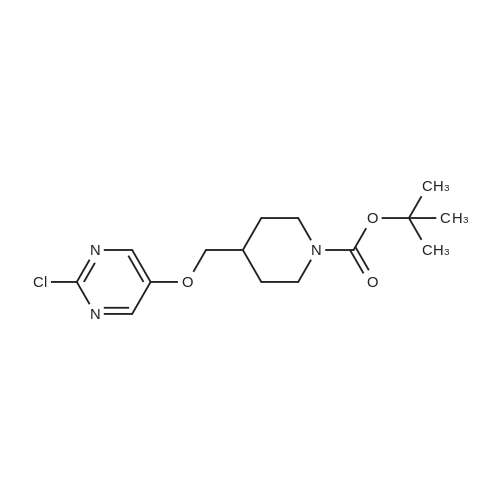
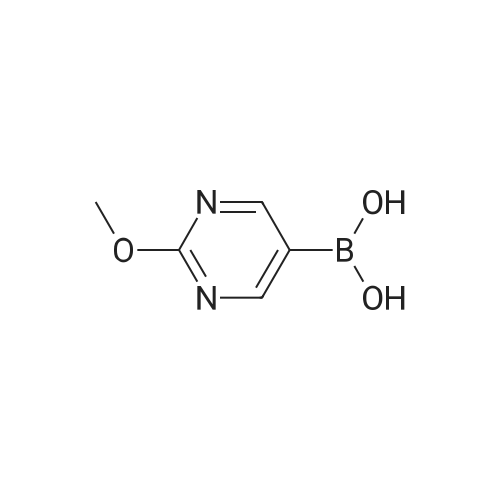
| 32 mg |
|
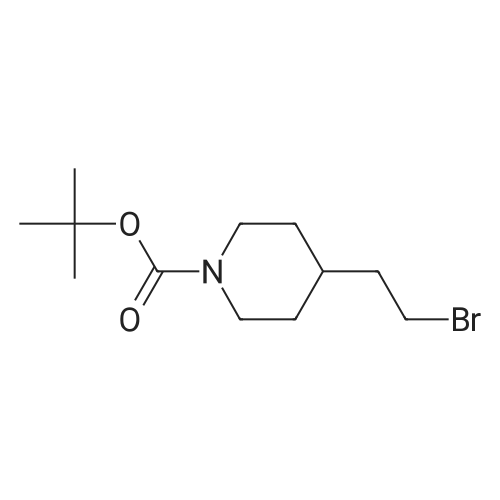
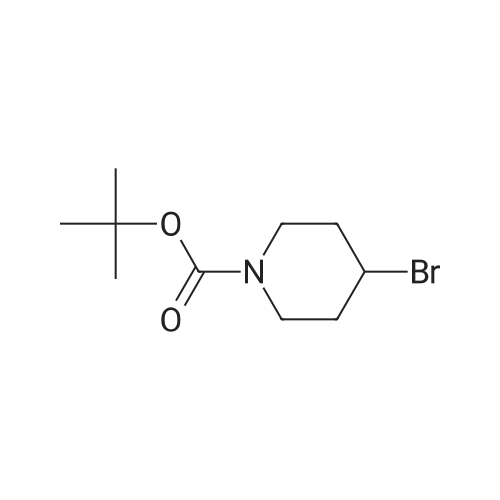
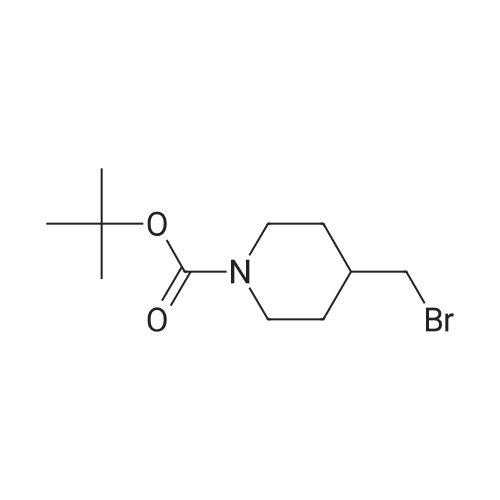
A mixture of <strong>[628692-15-9](2-methoxypyrimidin-5-yl)boronic acid</strong> (48 mg, 0.315 mmol), 3-(l-(4-methoxybenzyl)- lH-pyrazol-4-yl)-4,5,7,8-tetrahydro-lH-oxepino[4,5-c]pyrazole (100 mg) and DMAP (75 mg, 0.617 mmol) were combined in MeCN (1 mL) and treated with copper (II) acetate (84 mg, 0.462 mmol). The reaction mixture was stirred for three days at 40 °C, open to air, then allowed to cool to room temperature. The material was mixed with a batch that had been similarly prepared using (2-methoxypyrimidin-5- yl)boronic acid (131 mg, 0.848 mmol), 3-(l-(4-methoxybenzyl)-lH-pyrazol-4-yl)-4,5,7,8-tetrahydro- lH-oxepino[4,5-c]pyrazole (250 mg, 0.771 mmol), DMAP (188 mg, 1.541 mmol) and copper (II) acetate (210 mg, 1.156 mmol) in MeCN (5 mL).The combined reaction mixtures were treated with ammonium hydroxide (10 mL), water (10 mL) and partitioned with EtOAc (15 mL). The organic layer was isolated, and the aqueous layer re-extracted with EtOAc (2 x 15 mL). The combined organic layer was washed with brine (15 mL), passed through a hydrophobic frit and concentrated under reduced pressure, then loaded in MeOH (4 mL) onto an SCX-SPE cartridge that had been pre-conditioned with MeOH. The SCX-SPE cartridge was eluted with MeOH (3 x 15 mL) and combined eluants concentrated under reduced pressure to give a crude mixture the title compounds (308 mg). LCMS (Method C): Rt = 0.97 min, MH+ 433. Crude material was taken forward to the next reaction step without further purification. Intermediate 13. 2-(2-Methoxypyrimidin-5-yl)-3-(lH-pyrazol-4-yl)-4.5,7,8-tetrahydro- 2H-oxepinor4.5-c1pyrazole and l-(2-methoxypyrimidin-5-yl)-3-(lW-pyrazol-4-yl)- 4.5.7.8-tetrahydro-lH-oxepinor4.5-c1pyrazole A crude mixture of 3-(l-(4-methoxybenzyl)-lH-pyrazol-4-yl)-2-(2-methoxypyrimidin-5-yl)-4,5,7,8- tetrahydro-2H-oxepino[4,5-c]pyrazole and 3-(l-(4-methoxybenzyl)-lH-pyrazol-4-yl)-l-(2- methoxypyrimidin-5-yl)-4,5,7,8-tetrahydro-lH-oxepino[4,5-c]pyrazole (308 mg) in DCM (2 mL) was treated with TFA (2 mL, 26.0 mmol) and then heated using a microwave at 70 °C for 6 h. The reaction mixture was neutralised to pH 7 using sodium bicarbonate, diluted with water (10 mL) and extracted with EtOAc (15 mL). The organic layer was isolated, and the aqueous layer re-extracted with EtOAc (2 x 15 mL). The combined organic layer was washed with brine (10 mL), passed through a hydrophobic frit and concentrated under reduced pressure to give a crude mixture of the title compounds (232 mg). LCMS (Method C): Rt = 0.67 min, MH+ 313. Crude material was taken forward to the next reaction step without further purification. Intermediate 14. tert-Butyl 4-((4-(2-(2-methoxypyrimidin-5-yl)-4.5.7.8-tetrahydro-2W- oxepinor4.5-c1pyrazol-3-yl)-lW-pyrazol-l-yl)methyl)piperidine-l-carboxylate and tert- butyl 4-((4-(l-(2-methoxypyrimidin-5-yl)-4,5,7,8-tetrahydro-lH-oxepinor4,5-c1pyrazol- 3-yl)-lH-pyrazol-l-yl)methyl)piperidine-l-carboxylate A crude mixture of the 2-(2-methoxypyrimidin-5-yl)-3-(lH-pyrazol-4-yl)-4,5,7,8-tetrahydro-2H- oxepino[4,5-c]pyrazole and l-(2-methoxypyrimidin-5-yl)-3-(lH-pyrazol-4-yl)-4,5,7,8-tetrahydro-lH- oxepino[4,5-c]pyrazole (232 mg) was dissolved in DMF (2 mL) and placed under an atmosphere of nitrogen. The mixture was treated with NaH (17 mg, 0.423 mmol, 60percent suspension in mineral oils) and stirred for 30 min then treated with tert-butyl 4-(bromomethyl)piperidine-l-carboxylate (86 mg, 0.310 mmol) and stirred for a further 24 h. The reaction mixture was acidified to pH 5 using a 2 M solution of HCI and diluted with water (25 mL) then partitioned with EtOAc (25 mL). The organic layer was isolated and the aqueous layer re-extracted with EtOAc (25 mL). The combined organic layer was washed with LiCI (5percent solution in water, 2 x 25 mL) and brine (25 mL), and passed through a hydrophobic frit then concentrated under reduced pressure to give a crude mixture of the title compounds (90 mg). LCMS (Method A): Rt = 1.08 min, MH+ 510. Crude material was taken forward to the next reaction step without further purification. Intermediate 15. 2-(2-Methoxypyrimidin-5-yl)-3-(l-(piperidin-4-ylmethyl)-lH-pyrazol-4-yl)-4,5.7,8-tetrahydro-2H-oxepinor4,5-c1pyrazole and l-(2-methoxypyrimidin-5-yl)-3-(l-(piperidin-4-ylmethyl)-lH-pyrazol-4-yl)-4,5,7,8-tetrahydro-lH-oxepinor4,5- clpyrazole A mixture of tert-butyl 4-((4-(l-(2-methoxypyrimidin-5-yl)-4,5,7,8-tetrahydro-lH-oxepino[4,5- c]pyrazol-3-yl)-lH-pyrazol-l-yl)methyl)piperidine-l-carboxylate and tert-butyl 4-((4-(2-(2- methoxypyrimidin-5-yl)-4,5,7,8-tetrahydro-2H-oxepino[4,5-c]pyrazol-3-yl)-lH-pyrazol-l- yl)methyl)piperidine-l-carboxylate (90 mg) was dissolved in DCM (2 mL). The reaction mixture was placed under an atmosphere of nitrogen and treated with TFA (0.068 mL, 0.883 mmol) then stirred for 1 h. The reaction mixture was treated with saturated aqueous sodium carbonate solution (5 mL) and partitioned with EtOAc (15 mL). The organic layer was isolated and the aqueous layer re-extracted with EtOAc (2 x 15 mL). The combined organic layer wa... |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping