|
With iron; ammonium chloride; In water; at 90℃; for 1.25h; |
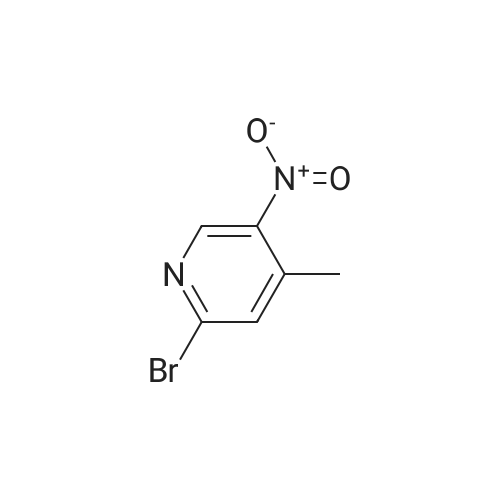
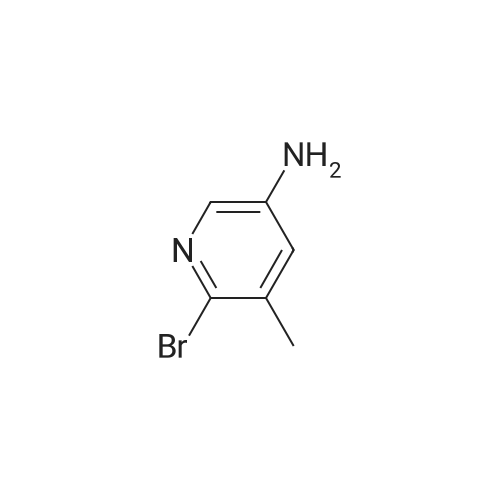
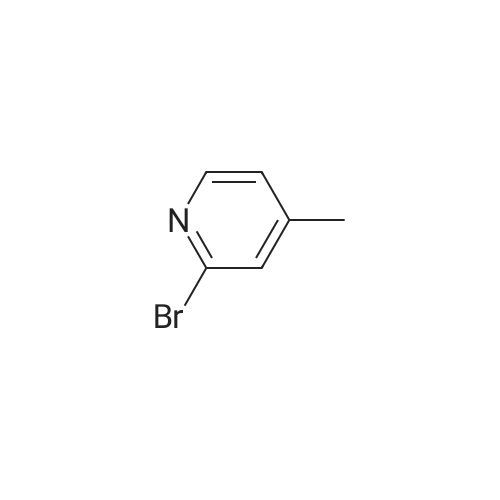
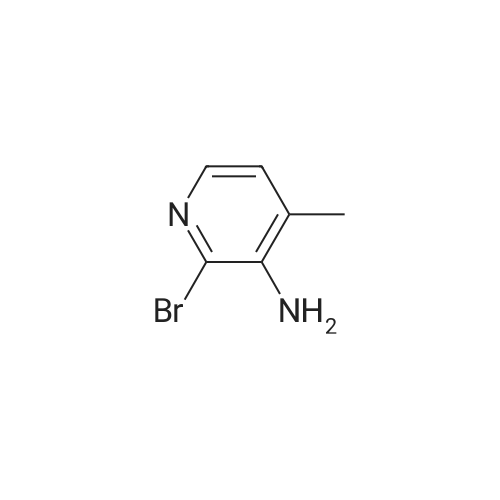
1.12. Synthesis of 6-bromo-4-methyl-pyridin-3-ylamine (Int.20) A mixture of NH4Cl (14.9 mmol) and iron powder (18.4 mmol) in H2O (5 mL) was stirred at 90 C. 2-bromo-4-methyl-5-nitropyridine (2.3 mmol) was added in portions. The mixture was stirred at 90 C. for 1 h and 15 min. The reaction was stopped and extracted with EtOAc. The organic layer was dried (Na2SO4) and concentrated to yield the desired product. |
|
With iron; ammonium chloride; In water; at 90℃; for 1.25h; |
1.12. Synthesis of 6-bromo-4-methyl-pyridin-3-ylamine (mt. 20) 1002591 A mixture of NH4C1 (14.9 mmol) and iron powder (18.4 mmol) in H20 (5 mL) was stirred at90C. 2-bromo-4-methyl-5-nitropyridine (2.3 mmol) was added in portions. The mixture was stirred at90C for 1 h and 15 mm. The reaction was stopped and extracted with EtOAc. The organic layer wasdried (Na2SO4) and concentrated to yield the desired product. |
|
With iron; ammonium chloride; In water; at 90℃; for 1.25h; |
A mixture of NH4C1 (14.9 mmol) and iron powder (18.4 mmol) in H20 (5 mE) was stirred at 90 C. 2-bromo-4- methyl-5-nitropyridine (2.3 mmol) was added in portions. The mixture was stirred at 90 C. for 1 h and 15 mm. The reaction was stopped and extracted with EtOAc. The organic layer was dried (Na2SO4) and concentrated to yield the desired product. |
|
With hydrogenchloride; iron; ammonium chloride; In ethanol; water; at 50 - 80℃; for 3h; |
Step 1 [0610] A suspension of 2-bromo-4-methyl-5-nitropyridine (XIV) (200 g, 921 mmol, 1.00 eq) and NH4C1 (240 g, 4.49 mol, 4.87 eq) in EtOH (3.50 L) and water (150 mL) was heated with stirring to 50C. To this mixture was added Fe ( 120 g, 2.15 mol, 2.33 eq) and HC1 (10.2 g, 279 mmol, 0.30 eq). The suspension was then heated to 80C for another 3 h. The reaction was cooled to 25C and filtered through a plug of Celite. The filtrate was concentrated under reduced pressure to yield a residue that was taken up in EtOAc (1 L x 3) and washed with brine. The organic layer was dried over sodium sulfate, filtered and concentrated under reduced pressure to give 6-bromo-4-methylpyridin-3 -amine (XV) as brown solid (167.9 g, 898 mmol, 97.4% yield) which was used for the next step without any purification. l NMR (CDC , 400 MHz) delta ppm 2.15 (s, 3H), 3.44 (br s, 2H), 7.14 (s, 1H), 7.78 (s, 1H); ESIMS found for C6H7BrN2 mlz 186.8 (M+H). |
|
With hydrogenchloride; iron; ammonium chloride; In ethanol; water; at 50 - 80℃; for 3h; |
Step 1 [0611] A suspension of 2-bromo-4-methyl-5-nitropyridine (XIV) (200 g, 921 mmol, 1.00 eq) and NH4C1 (240 g, 4.49 mol, 4.87 eq) in EtOH (3.50 L) and water (150 mL) was heated with stirring to 50C. To this mixture was added Fe (120 g, 2.15 mol, 2.33 eq) and HC1 (10.2 g, 279 mmol, 0.30 eq). The suspension was then heated to 80C for another 3 h. The reaction was cooled to 25C and filtered through a plug of Celite. The filtrate was concentrated under reduced pressure to yield a residue that was taken up in EtOAc (1 L x 3) and washed with brine. The organic layer was dried over sodium sulfate, filtered and concentrated under reduced pressure to give 6-bromo-4-methylpyridin-3 -amine (XV) as brown solid (167.9 g, 898 mmol, 97.4% yield) which was used for the next step without any purification. l NMR (CDC , 400 MHz) delta ppm 2.15 (s, 3H), 3.44 (br s, 2H), 7.14 (s, 1H), 7.78 (s, 1H); ESIMS found for C6H7BrN2 mlz 186.8 (M+H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping