| 77% |
|
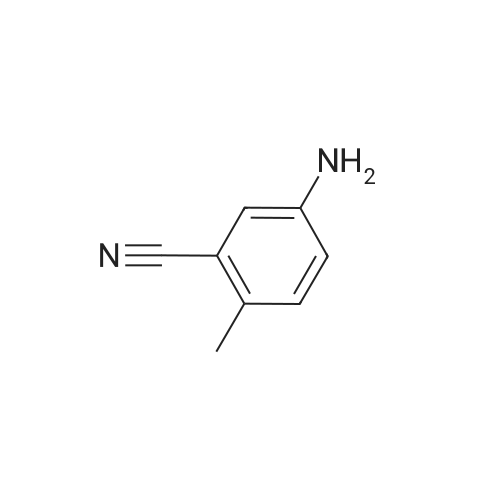
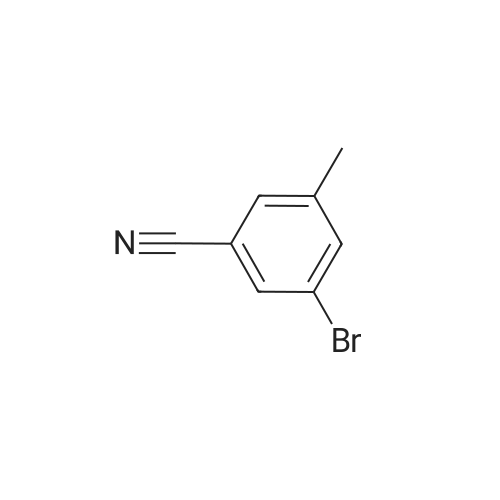
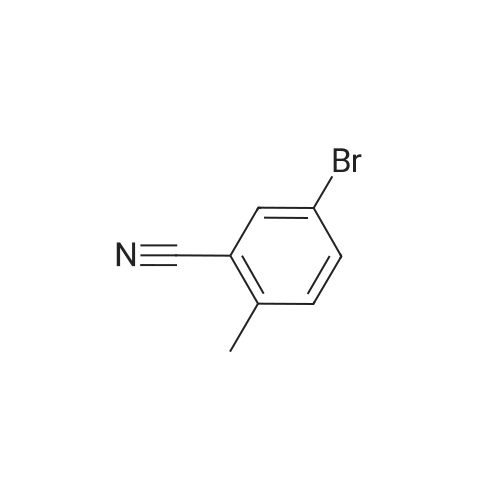
Example 22 5-Bromo-2-methylbenzonitrile Water (13.5 mL), HBr (74%, 14.4 mL) and <strong>[50670-64-9]5-amino-2-methylbenzonitrile</strong> (2.0 g, 15.1 mmol) dissolved in water (24 mL) was added to a flask and heated to 50 C. for 20 min. Then the mixture was cooled to 0~5 C., and a solution of NaNO2 (1.2 g, 17.4 mmol) in water was added. The reaction mixture was stirred for 10 min at 0~5 C., then was warmed to 40 C. A solution of CuBr (6.5 g, 45.1 mmol) in water (36 mL) and HBr (7.2 mL) was added to the mixture, and refluxed for 2 h. The mixture was extracted with AcOEt, and the organic layer was washed by saturated NaHCO3 solution and brine, and dried over Na2SO4. The crude product was purified by flask chromatograph (PE:EA=50:1), obtaining 2.3 g of 5-bromo-2-methylbenzonitrile as a white solid (yield: 77%). 1H NMR (400 MHz, CDCl3) delta 7.72 (s, 1H), 7.59 (d, J=8.0 Hz, 1H), 7.19 (d, J=8.0 Hz, 1H), 2.51 (s, 3H). |
| 77% |
|
Example 22 5-Bromo-2-methylbenzonitrile Water (13.5 mL), HBr (74%, 14.4 mL) and <strong>[50670-64-9]5-amino-2-methylbenzonitrile</strong> (2.0 g, 15.1 mmol) dissolved in water (24 mL) was added to a flask and heated to 50 C. for 20 min. Then the mixture was cooled to 0~5 C., and a solution of NaNO2 (1.2 g, 17.4 mmol) in water was added. The reaction mixture was stirred for 10 min at 0~5 C., then was warmed to 40 C. A solution of CuBr (6.5 g, 45.1 mmol) in water (36 mL) and HBr (7.2 mL) was added to the mixture, and refluxed for 2 h. The mixture was extracted with AcOEt, and the organic layer was washed by saturated NaHCO3 solution and brine, and dried over Na2SO4. The crude product was purified by flask chomatograph (PE:EA=50:1), obtaining 2.3 g of 5-bromo-2-methylbenzonitrile as a white solid (yield: 77%). 1H NMR (400 MHz, CDCl3) delta 7.72 (s, 1H), 7.59 (d, J=8.0 Hz, 1H), 7.19 (d, J=8.0 Hz, 1H), 2.51 (s, 3H). |
| 77% |
|
Example 25 5-Bromo-2-methylbenzonitrile Keep the solution of <strong>[50670-64-9]5-amino-2-methylbenzonitrile</strong> (2.0 g, 15.1 mmol) dissolved in water (24 mL) and HBr (74%, 14.4 mL) stirring for 20 min, then the mixture was cooled to 0~5 C., and the solution of NaNO2 (1.2 g, 17.4 mmol) in water (13.5 mL) was added. The reaction mixture was stirred for 10 min at 0~5 C., then was warmed to 40 C. A solution of CuBr (6.5 g, 45.1 mmol) in water (36 mL) and HBr (7.2 mL) was added to the mixture, and it was stirred for 2 h. The mixture was extracted with AcOEt, and the organic layer was washed with saturated NaHCO3 solution, brine, and dried over Na2SO4. The crude product was purified by flask chromatograph (PE:EA=50:1), obtaining 2.3 g of 5-bromo-2-methylbenzonitrile as a white solid (Yield: 77%). 1H NMR (400 MHz, CDCl3): delta 7.72 (s, 1H), 7.59 (d, J=8.0 Hz, 1H), 7.19 (d, J=8.0 Hz, 1H), 2.51 (s, 3H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping