|
at 80℃; |
The PPS-TPAs IL catalysts were prepared following the procedures outlined in the literature (see Scheme S1; Supplementary data) [32,49]. In brief, known amounts of 1,3-propane sultone (12.2 g, 0.1 mol) was first mixed with pyridine (7.91 g, 0.1 mol) under stirring condition at 80 C for about 6-12 h. Then, the solid zwitterion mass was filtered and washed thrice with ethyl acetate, followed by drying under vacuum (?76 Torr). Subsequently, an aqueous solution of TPA (H3PW12O40; 5.76 g in 50 mL H2O) was added, and the mixture was stirred at 90 C for 24 h. After removal of water, the final product was washed with diethyl ether, and then dried under vacuum. A series of PPS-TPA IL salts so prepared with varied PPS/TPA ratios are denoted as [PPSH]xH3-xPW12O40; x = 1.0-3.0. All research grade chemicals were used without further purification. |
|
In toluene; at 50℃; for 24.0h;Inert atmosphere; |
General procedure: Methylimidazole (0.11 mol) and 1,3-propane sulfone (0.10 mol) were dissolved in toluene (30mL) and stirred for 24 h at 50 C under a nitrogen atmosphere. A white precipitate 3-(1-methyl)imidazolium propanesulfonate (MIMPS) formed,which was filtered,washed with diethyl ether three times, then dried in a vacuum. MIMPS (0.09 mol) was added to an aqueous solution of H3PW12O40 (0.03mol), and then the mixture was stirred at room temperature for 24 h. Water was removed in vacuum to give the product [MIMPS]3PW12O40 as a solid. |
|
In toluene; at 50℃; for 24.0h;Inert atmosphere; |
Toa mixture of toluene (30 mL) and 1,3-propane sulfone (0.10 mol, 12.2 g) in a 100 mLround bottomed flask was added pyridine (0.11 mol, 8.7 g, 8.9 mL). The reactionmixture was stirred for 24 h at 50oC under a nitrogen atmosphereuntil a white precipitate (PyPS) was formed. After filtration, washing withdiethyl ether and drying in a vacuum, PyPS was obtained. To an aqueous solutionof H3PW12O40 (0.03 mol, 86.4 g) was added PyPS(0.09 mol, 18.1 g) and then the mixture was stirred at room temperature for 24h. Finally, the solution was removed in vacuum to give the product [PyPS]3PW12O40as a solid. Thus [MIMPS]3PW12O40, [MIMPS]3PMo12O40,[PyPS]3PMo12O40, [TEAPS]3PW12O40and [TEAPS]3PMo12O40 were prepared usingaccording starting materials. |
|
In acetone; at 50℃; for 4.0h; |
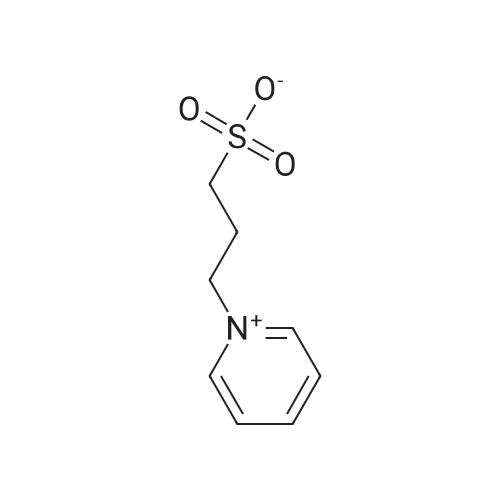
1-(3-sulfonic group) propyl-pyridine (abbreviated as PyPS) and 1-(3-sulfonic group) propyl-triethylammonium (abbreviated as TEAPS) were synthesized according to the literature [8]. Pyridine (0.055 mol) and 1,3-propanesultone (0.05 mol) were dissolved inacetone (25 ml) and stirred at 50 C for 4 h. A white precipitate (PyPS) was obtained and washed by acetone for three times, thendried under vacuum. Similarly, TEAPS can be prepared. |
|
In toluene; at 50℃; for 24.0h;Inert atmosphere; |
To a well-stirred mixture of 12.2 g 1,3-propane sulfone (0.10 mol) in 30 mL toluene were added 8.9 mL pyridine (0.11 mol). The reaction mixture was stirred for 24 h at 50 C under a nitrogen atmosphere resulting a white precipitate (PyPS). After the completion of reaction, it was cooled to room temperature. PyPS was obtained after filtration, washing with diethyl ether and drying in a vacuum. And then 18.1 g PyPS (0.09 mol) was added to an aqueous solution of 86.4 g H3PW12O40 (0.03 mol). After stirring at room temperature for 24 h, the solution was removed in vacuum to give the HPAIL product [PyPS]3PW12O40 as a solid. Thus [PyPS]3PMo12O40, [MIMPS]3PW12O40, [MIMPS]3PMo12O40, [TEAPS]3PW12O40 and [TEAPS]3PMo12O40 were prepared using according starting materials. 4.2.1. 1-(3-Sulfopropyl)pyridine (PyPS) White solid. Mp: 62.5-64.8 C; IR (KBr): 3443, 3159, 3103, 1572, 1459, 1184, 1010, 842, 780, 654, 601, 528 cm-1; 1H NMR (400 MHz, D2O) delta 8.86 (d, J = 6.0 Hz, 2H), 8.53 (t, J = 7.8 Hz, 1H), 8.06 (t, J = 7.6 Hz, 2H), 4.75 (t, J = 7.2 Hz, 2H), 2.95 (t, J = 7.2 Hz, 2H), 2.47-2.40 (m, 2H); 13C NMR (100 MHz, D2O) delta 146.0, 144.5, 128.5, 60.0, 47.1, 26.2; HRMS Calcd for C8H12NO3S (M+H+): 202.0532; Found: 202.0534. |
|
at 40℃; for 24.0h;Inert atmosphere; |
Take 0.1 mol pyridine in four flask at constant pressure dropping funnel equimolar 1,3-propane sultone, at 40 C, N 2 protection conditions, stirring 24 h, to give a white precipitate intermediate , washed three times with ethyl acetate and dried in vacuo 8 h, to give a white intermediate: 0.1 mol 1- (3- sulfopropyl) pyridinium inner salt of PPS. Under ice-cooling, to the PPS of concentrated sulfuric acid was added dropwise an equimolar, to warm slowly After the addition the reaction to 40 C 72 h, until a white solid was dissolved, washed with diethyl ether and dried in vacuo to obtain the ionic liquid [PSpy] HSO 4. Prepared as described above was weighed [PSpy] HSO 4 Catalyst 5 mmol, glycerol 1.0 mol, stirred and heated to 250 C, the reaction carried out batchwise, react sufficiently until no distillate produced, sampled for gas chromatographic analysis. Analysis concluded that glycerin conversion was 100% and the yield of acrolein of 48.7%. |
|
at 40℃; for 24.0h; |
Please refer to FIG. 2 and FIG. 3, which are a view showing reactions of fabricating the IL; and a view showing transacylation and stratification. As shown in the figures, on using the present invention, for fabricating an acidic IL as a catalyst, pyridine or 1-butyl-imidazole (N-butyl imidazole) is reacted with 1,3-propane sultone at 40 C. for 24 hrs to obtain a white solid of a zwitterionic compound. After being purified with ether and dried through vacuuming, the white solid of the zwitterionic compound of R+-(CH2)3-SO3- is obtained, where R is pyridine or 1-butyl-imidazole (i.e. n-propane sulfonic acid pyridinium (PSPy) or pyridinium propyl sulfobetaine (PPS)). An appropriate amount of the white solid is obtained in a round bottom flask to be added with a considerable number of moles of sulfuric acid for reaction with stirring at 100 C. for 0.5 hr. A transparent viscous liquid is gradually formed, which is an acidic IL of [R+-(CH2)3-SO3H][HSO4] obtained through the reaction shown in FIG. 2. |
|
In toluene; at 20℃; for 4.0h;Cooling with ice; |
Preparation of modified acidic functional ionic liquid [C3SO3HPy] HSO4 · 0.2MgCl21, (124.6 g, 1.0 mol) l, 3-propanesultone was dissolved in 500 mL of toluene with rapid stirring,After dissolving, the system was placed in an ice-water bath; slowly (79.1 g, 1.0 mol) of pyridine was added dropwise and the reaction was allowed to react in an ice bath for 2 hAfter that, go to room temperature for 2 h;2, after the end of the reaction, the resulting white solid was filtered under reduced pressure and washed three times with anhydrous ether; the solid was placed in realEmpty oven, vacuum drying at 80 C for 24 h. To obtain a C3SO3-Py solid;3. The C3SO3-Py solid (204.2 g, 1.0 mol) was dissolved in 500 ml of distilled water and slowly added dropwise at room temperature(100.1 g, 1.0 mol) of 98% concentrated sulfuric acid, and then the system was heated to 60 C for 2 h;4. After completion of the reaction, (18.9 g, 0.2 mol) of magnesium chloride was added to the resulting solution, and the reaction was stirred at 60 Cminute. After completion of the reaction, most of the water was removed by steaming at 70 C under reduced pressure, and the mixture was further charged in a vacuum oven at 80 CAfter drying for 24 h, 321.1 g of [C3SO3HPy] HSO4 · 0.2MgCl2 viscous liquid was obtained. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping