| 77% |
With hydrazine hydrate; In tetrahydrofuran; |
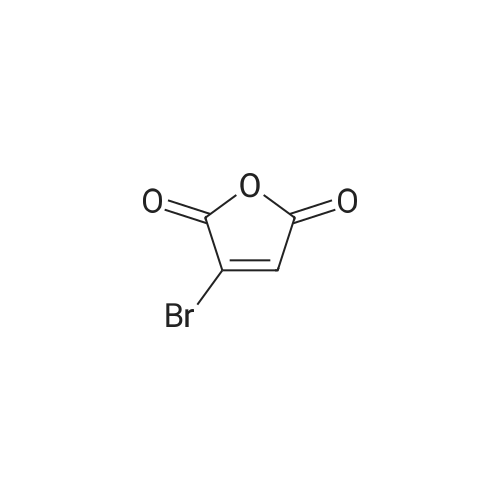
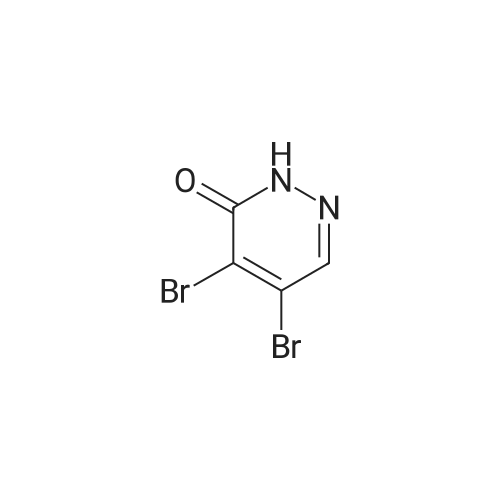
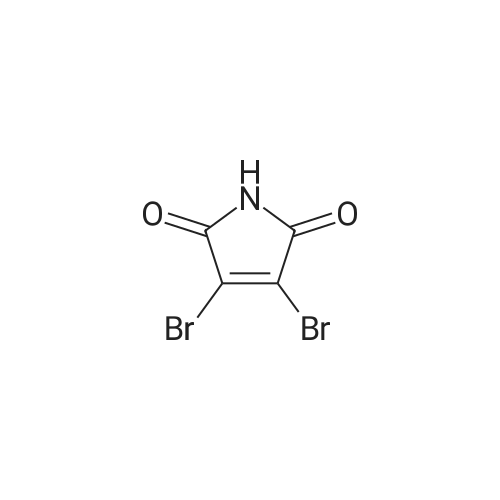
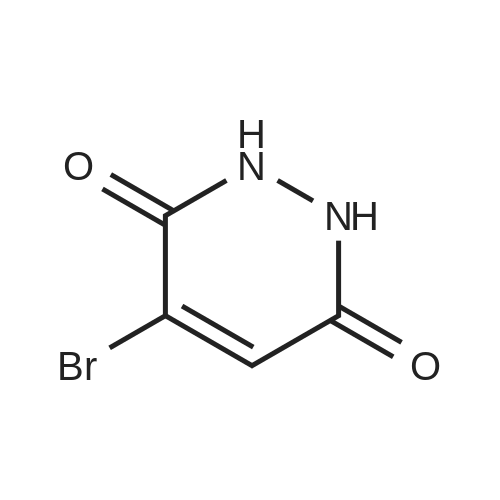
a 4-Bromo-1,2-dihydropyridazine-3,6-dione Hydrazine hydrate (28 ml, 576 mmol) was added dropwise to a stirred solution of <strong>[5926-51-2]bromomaleic anhydride</strong> (100 g, 565 mmol) in THF (1 l) cooled in an ice-bath so that the internal temperature did not exceed 10° C. After complete addition of the hydrazine the mixture was refluxed for 18 h. Solvent was removed by evaporation and the residues were dried by azeotroping with toluene. The residue was triturated and washed with diethyl ether to give the title compound as an orange solid (83 g, 77percent). 1H NMR (250 MHz, d6-DMSO) delta 7.68 (1H, br s). MS (ES+) m/e 193 [MH]+, 191 [MH]+. This material was used without further purification. |
| 56% |
With (x)H2O4S*H4N2; In water; at 90℃; for 4h; |
To a solution of NH2NH2?H2504 (362 mg, 2.8 mmol) in H20 (5 mL) was added dropwise <strong>[5926-51-2]3-bromofuran-2,5-dione</strong> (500 mg, 2.8 mmol), and the mixture was stirred at 90 °C for 4hours. After the reaction, the mixture was filtered to get 4-bromo-1,2-dihydropyridazine-3,6- dione (300 mg, yield: 56percent). ?H-NMR (DMSO-d6, 400 MHz) 12.47 (s, 1H), 11.17 (s, 1H), 7.62 (s, 1H). MS (M+H): 191 / 193. |
| 56% |
With hydrazinium sulfate; In water; at 90℃; for 4h; |
To a solution ofNH2NH2?H2S04 (362 mg, 2.8 mmol) in H20 (5 mL) was addeddropwise <strong>[5926-51-2]3-bromofuran-2,5-dione</strong> (500 mg, 2.8 mmol), and the mixture was stirred at 90 °C for 410 hours. After the reaction, the mixture was filtered to get 4-bromo-1,2-dihydropyridazine-3,6-dione (300 mg, yield: 56percent). 1H-NMR (DMSO-d6, 400 MHz) 8 12.47 (s, 1H), 11.17 (s, 1H),7.62 (s, 1H). MS (M+Ht: 191 I 193. |
| 37% |
With sodium acetate; hydrazine hydrate; In water; acetic acid; |
a) 4-Bromo-1,2-dihydropyridazine-3,6-dione A mixture of <strong>[5926-51-2]bromomaleic anhydride</strong> (50 g, 283 mmol) and sodium acetate (76.5 g, 562 mmol) in 40percent acetic acid/water (750 ml) was treated with hydrazine monohydrate (16.5 ml, 339 mmol) at room temperature under nitrogen. The brown solution was stirred and heated at 100° C. for 18 hours. Upon cooling the mixture was poured into water (1 l) and extracted with ethyl acetate (6*500 ml). The combined extracts were dried (MgSO4), filtered and evaporated to afford the title pyridazine (20 g, 37percent) as an orange solid. 1H NMR (250 MHz, d6-DMSO) 7.68 (br s). MS (ES+) 193 [MH]+, 191 [MH]+. This material was used without further purification. |
| 37% |
With sodium acetate; hydrazine hydrate; In water; acetic acid; |
a 4Bromo-1,2-dihydropyridazine-3,6-dione A mixture of <strong>[5926-51-2]bromomaleic anhydride</strong> (50 g, 283 mmol) and sodium acetate (76.5 g, 562 mmol) in 40percent acetic acid/water (750 ml) was treated with hydrazine monohydrate (16.5 ml, 339 mmol) at room temperature under nitrogen. The brown solution was stirred and heated at 100° C. for 18 hours. Upon cooling the mixture was poured into water (1 l) and extracted with ethyl acetate (6*500 ml). The combined extracts were dried (MgSO4), filtered and evaporated to afford the title compound (20 g, 37percent) as an orange solid. 1H NMR (250 MHz, d6-DMSO) delta 7.68 (1H, br s). MS (ES+) m/e 193 [MH]+, 191 [MH]+. This material was used without further purification. |
| 37% |
With sodium acetate; hydrazine hydrate; In water; acetic acid; |
a 4-Bromo-1,2-dihydropyridazine-3,6-dione A mixture of <strong>[5926-51-2]bromomaleic anhydride</strong> (50 g, 283 mmol) and sodium acetate (76.5 g, 562 mmol) in 40percent acetic acid/water (750 ml) was treated with hydrazine monohydrate (16.5 ml, 339 mmol) at room temperature under nitrogen. The brown solution was stirred and heated at 100° C. for 18 hours. Upon cooling the mixture was poured into water (11) and extracted with ethyl acetate (6*500 ml). The combined extracts were dried (MgSO4), filtered and evaporated to afford the title compound (20 g, 37percent) as an orange solid. 1H NMR (250 MHz, d6-DMSO) delta7.68 (1H, br s). MS (ES+) m/e 193 [MH]+, 191 [MH]+. This material was used without further purification. |
|
With sodium acetate; hydrazine hydrate; In water; acetic acid; |
a 4-Bromo-1,2-dihydropyridazine-3,6-dione A mixture of <strong>[5926-51-2]bromomaleic anhydride</strong> (50 g, 283 mmol) and sodium acetate (76.5 g, 562 mmol) in 40percent acetic acid/water (750 ml) was treated with hydrazine monohydrate (16.5 ml, 339 mmol) at room temperature under nitrogen. The brown solution was stirred and heated at 100° C. for 18 h. Upon cooling the mixture was poured into water (1 l) and extracted with ethyl acetate (6*500 ml). The combined extracts were dried (MgSO4), filtered and evaporated to afford the title pyridazile (20 g, 37percent) as an orange solid. 1H NMR (250 MHz, d6-DMSO) 7.68 (br s). MS (ES30) 193 [MH]+, 191 [MH]+. This material was used without further purification. |
|
With hydrazinium sulfate; In water; at 20℃; for 4h;Heating / reflux; |
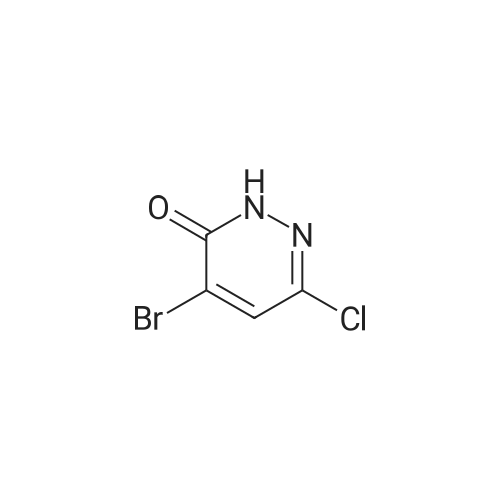
(a) 3,4,6-TrichloropyridazineThis was prepared by a slight variation on the method of Kasnar et al,Nucleosides Nucleotides (1994), 13(1-3), 459-79. Hydrazine sulphate salt (51 g) was suspended in water (250ml), heated to reflux and <strong>[5926-51-2]bromomaleic anhydride</strong> (90.38 g) was added dropwise . The mixture was heated at reflux for 4 hours then cooled to room temperature. The reaction was repeated with 29g hydrazine sulphate, 53g <strong>[5926-51-2]bromomaleic anhydride</strong> and 130ml water.The precipitates were collected by filtration, washed with water and acetone and dried as a combined batch in vacuo to afford 4-bromo-l,2-dihydro-3,6-pyridazinedione as a white solid (113 g). |
|
With hydrazinium sulfate; In water; for 4h;Heating / reflux; |
Examples 3 and 4 5-({4-[(6,7-Dihydro[l,4]dioxino[2,3-c]pyridazin-3- ylmethyl)amino] -1 -piperidinyl} methyl)-3-fluoro-4,5-dihydro-7H-pyrrolo [3,2,1 -de] - l,5-naphthyridin-7-one hydrochloride, Enantiomers 1 and 2(a) 3,4,6-TrichloropyridazineThis was prepared by a slight variation on the method of Kasnar et al, Nucleosides Nucleotides (1994), 13(1-3), 459-79. Hydrazine sulphate salt (51 g) was suspended in water (250ml), heated to reflux and <strong>[5926-51-2]bromomaleic anhydride</strong> (90.38 g) was added dropwise . The mixture was heated at reflux for 4 hours then cooled to room temperature. The reaction was repeated with 29g hydrazine sulphate, 53g <strong>[5926-51-2]bromomaleic anhydride</strong> and 130ml water. The precipitates were collected by filtration, washed with water and acetone and dried as a combined batch in vacuo to afford 4-bromo-l,2-dihydro-3,6-pyridazinedione as a white solid (113 g).The solid in two batches was treated with phosphorus oxychloride (2x200 ml) and heated to reflux for 3.5 hours. The mixture was cooled, evaporated and azeotroped with toluene. The residue was partitioned between dichloromethane and saturated aqueous sodium bicarbonate solution and extracted with DCM twice more. The organic extracts were dried and evaporated. This residue was re-dissolved in dichloromethane, and chromatographed on silica gel (300 g) (DCM as eluent) to give a white solid (101.5 g,percent}.(LC/MS analysis showed ca 20-30percent impurity, isomers of bromo-dichloropyridazine). MS (+ve ion electrospray) m/z 184/185/186 (MH+), trichloropyridazine. MS (+ve ion electrospray) m/z 228/229/231 (MH+), bromo-dichloropyridazine. |
|
With hydrazinium sulfate; In water; for 4h;Heating / reflux; |
This was prepared by a slight variation on the method of Kasnar et al, Nucleosides Nucleotides, (1994), 13(1-3), 459-79. Hydrazine sulphate salt (51 g) was suspended in water (250ml), heated to reflux and <strong>[5926-51-2]bromomaleic anhydride</strong> (90.38 g) was added dropwise. The mixture was heated at reflux for 4 h then cooled to room temperature. The reaction was repeated with 29 g hydrazine sulphate, 53 g <strong>[5926-51-2]bromomaleic anhydride</strong> and 130ml water. The precipitates were collected by filtration, washed with water and acetone and dried as a combined batch in vacuo to afford 4-bromo-l ,2- dihydro-3,6-pyridazinedione as a white solid (113 g). |
|
With hydrazinium sulfate; In water;Reflux; |
c) 3,4,6-Trichloropyridazine; This was prepared by a slight variation on the method of Kasnar et al, Nucleosides Nucleotides (1994), 13(1-3), 459-79.Hydrazine sulphate salt (51 g) was suspended in water (250 ml), heated to reflux and <strong>[5926-51-2]bromomaleic anhydride</strong> (90.38 g) was added dropwise. The mixture was heated at reflux for 4 hours then cooled to room temperature. The reaction was repeated with 29 g hydrazine sulphate, 53 g <strong>[5926-51-2]bromomaleic anhydride</strong> and 130 ml water. The precipitates were collected by filtration, washed with water and acetone and dried as a combined batch in vacuo to afford 4-bromo-1,2-dihydro-3,6-pyridazinedione as a white solid (113 g). The solid in two batches was treated with phosphorus oxychloride (2.x.200 ml) and heated to reflux for 3.5 hours. The mixture was cooled, evaporated and azeotroped with toluene. The residue was partitioned between dichloromethane and saturated aqueous sodium bicarbonate solution and extracted with DCM twice more. The organic extracts were dried and evaporated. This residue was re-dissolved in dichloromethane, and chromatographed on silica gel (300 g) (DCM as eluent) to give a white solid (101.5 g, 87percent).(LC-MS analysis showed ca 20-30percent impurity, isomers of bromo-dichloropyridazine).MS (+ve ion electrospray) m/z 184/185/186 (MH+), trichloropyridazine.MS (+ve ion electrospray) m/z 228/229/231 (MH+), bromo-dichloropyridazine. |
|
With hydrazine sulphate; In water; for 4h;Heating / reflux; |
a) 3,4,6-TrichloropyridazineThis was prepared by a slight variation on the method of Kasnar et al, Nucleosides Nucleotides (1994), 13(1-3), 459-79.Hydrazine sulphate salt (51 g) was suspended in water (250 ml), heated to reflux and <strong>[5926-51-2]bromomaleic anhydride</strong> (90.38 g) was added dropwise. The mixture was heated at reflux for 4 hours then cooled to room temperature. The reaction was repeated with 29 g hydrazine sulphate, 53 g <strong>[5926-51-2]bromomaleic anhydride</strong> and 130 ml water. The precipitates were collected by filtration, washed with water and acetone and dried as a combined batch in vacuo to afford 4-bromo-1,2-dihydro-3,6-pyridazinedione as a white solid (113 g).The solid in two batches was treated with phosphorus oxychloride (2.x.200 ml) and heated to reflux for 3.5 hours. The mixture was cooled, evaporated and azeotroped with toluene. The residue was partitioned between dichloromethane and saturated aqueous sodium bicarbonate solution and extracted with DCM twice more. The organic extracts were dried and evaporated. This residue was re-dissolved in dichloromethane, and chromatographed on silica gel (300 g) (DCM as eluent) to give a white solid (101.5 g, 87percent).(LCAMS analysis showed ca 20-30percent impurity, isomers of bromo-dichloropyridazine).MS (+ve ion electrospray) m/z 184/185/186 (MH+), trichloropyridazineMS (+ve ion electrospray) m/z 228/229/231 (MH+), bromo-dichloropyridazine. |
|
With hydrazine sulphate; In water; for 4h;Heating / reflux; |
(a) 3,4,6-Trichloropyridazine; This was prepared by a slight variation on the method of Kasnar et al, Nucleosides Nucleotides, (1994), 13(1-3), 459-79. Hydrazine sulphate salt (51 g) was suspended in water (250ml), heated to reflux and <strong>[5926-51-2]bromomaleic anhydride</strong> (90.38 g) was added dropwise . The mixture was heated at reflux for 4 h then cooled to room temperature. The reaction was repeated with 29g hydrazine sulphate, 53g <strong>[5926-51-2]bromomaleic anhydride</strong> and 130ml water. The precipitates were collected by filtration, washed with water and acetone and dried as a combined batch in vacuo to afford 4-bromo-1 ,2-dihydro-3,6-pyridazinedione as a white solid (113 g). |
|
With hydrazinium sulfate; In water; for 4h;Heating / reflux; |
Hydrazine sulphate salt (51 g) was suspended in water (250ml), heated to reflux and <strong>[5926-51-2]bromomaleic anhydride</strong> (90.38 g) was added dropwise . The mixture was heated at reflux for 4 hours then cooled to room temperature. The reaction was repeated with 29g hydrazine sulphate, 53g <strong>[5926-51-2]bromomaleic anhydride</strong> and 130ml water. The precipitates were collected by filtration, washed with water and acetone and dried as a combined batch in vacuo to afford 4-bromo-l,2-dihydro-3,6-pyridazinedione as a white solid (113 g). |
|
With hydrazinium sulfate; In water; for 8h;Heating / reflux; |
Example 3; 3-({4- [(6,7-Dihydro [ 1 ,4] dioxino [2,3-c] pyridazin-3-ylmethyl)amino] - l-piperidinyl}methyl)-10-fluoro-2,3-dihydro-lH,5H-pyrido[3,2,l-//]quinolin-5- one dihydrochloride salt; (a) 3,4,6-Trichloropyridazine; <n="33"/>This was prepared by a slight variation on the method of Kasnar et al,Nucleosides Nucleotides (1994), 13(1-3), 459-79.Hydrazine sulphate salt (51 g) was suspended in water (250ml), heated to reflux and <strong>[5926-51-2]bromomaleic anhydride</strong> (90.38 g) was added dropwise . The mixture was heated at reflux for 4 hours then cooled to room temperature. The reaction was repeated with 29g hydrazine sulphate, 53g <strong>[5926-51-2]bromomaleic anhydride</strong> and 130ml water.The precipitates were collected by filtration, washed with water and acetone and dried as a combined batch in vacuo to afford 4-bromo-l,2-dihydro-3,6-pyridazinedione as a white solid (113 g). The solid in two batches was treated with phosphorus oxychloride (2x200 ml) and heated to reflux for 3.5 hours. The mixture was cooled, evaporated and azeotroped with toluene. The residue was partitioned between dichloromethane and saturated aqueous sodium bicarbonate solution and extracted with DCM twice more.The organic extracts were dried and evaporated. This residue was re-dissolved in dichloromethane, and chromatographed on silica gel (300 g) (DCM as eluent) to give a white solid (101.5 g, 87percent).(LC/MS analysis showed ca 20-30percent impurity, isomers of bromo-dichloropyridazine).MS (+ve ion electrospray) m/z 184/185/186 (MH+), trichloropyridazine.MS (+ve ion electrospray) m/z 228/229/231 (MH+), bromo-dichloropyridazine. |
|
With hydrazinium sulfate; In water; for 8h;Heating / reflux; |
(h) 3,4,6-Trichloropyridazine; This was prepared by a slight variation on the method of Kasnar et al, Nucleosides Nucleotides (1994), 13(1-3), 459-79.Hydrazine sulphate salt (51 g) was suspended in water (250ml), heated to reflux and <strong>[5926-51-2]bromomaleic anhydride</strong> (90.38 g) was added dropwise . The mixture was heated at reflux for 4 hours then cooled to room temperature. The reaction was repeated with 29 g hydrazine sulphate, 53 g <strong>[5926-51-2]bromomaleic anhydride</strong> and 130ml water. The precipitates were collected by filtration, washed with water and acetone and dried as a combined batch in vacuo to afford 4-bromo-l,2-dihydro-3,6-pyridazinedione as a white solid (113 g).The solid in two batches was treated with phosphorus oxychloride (2 x 200 ml) and heated to reflux for 3.5 hours. The mixture was cooled, evaporated and azeotroped with toluene. The residue was partitioned between dichloromethane and saturated aqueous sodium bicarbonate solution and extracted with DCM twice more. The organic extracts were dried and evaporated. This residue was re-dissolved in dichloromethane, and chromato graphed on silica gel (300 g) (DCM as eluent) to give a white solid (101.5(LC/MS analysis showed ca 20-30percent impurity, isomers of bromo-dichloropyridazine).MS (+ve ion electrospray) m/z 184/185/186 (MH+), trichloropyridazine.MS (+ve ion electrospray) m/z 228/229/231 (MH+), bromo-dichloropyridazine. |
|
With hydrazinium sulfate; In water; for 8h;Heating / reflux; |
Preparation A; 6,7-Dihydro [1 ,4] dioxino [2,3-c] pyridazine-3-carbaldehyde; (a) 3,4,6-Trichloropyridazine; This was prepared by a slight variation on the method of Kasnar et al,Nucleosides Nucleotides (1994), 13(1-3), 459-79.Hydrazine sulphate salt (51 g) was suspended in water (250ml), heated to reflux and <strong>[5926-51-2]bromomaleic anhydride</strong> (90.38 g) was added dropwise . The mixture was heated at reflux for 4 hours then cooled to room temperature. The reaction was repeated with 29g hydrazine sulphate, 53 g <strong>[5926-51-2]bromomaleic anhydride</strong> and 130ml water. The precipitates were collected by filtration, washed with water and acetone and dried as a combined batch in vacuo to afford 4-bromo-l,2-dihydro-3,6-pyridazinedione as a white solid (113 g).The solid in two batches was treated with phosphorus oxychloride (2x200 ml) and heated to reflux for 3.5 hours. The mixture was cooled, evaporated and azeotroped with toluene. The residue was partitioned between dichloromethane and saturated aqueous sodium bicarbonate solution and extracted with DCM twice more. The organic extracts were dried and evaporated. This residue was re-dissolved in dichloromethane, and chromatographed on silica gel (300 g) (DCM as eluent) to give a white solid (101.5 g, 87percent). (LC/MS analysis showed ca 20-30percent impurity, isomers of bromo-dichloropyridazine). MS (+ve ion electrospray) m/z 184/185/186 (MH+), trichloropyridazine. MS (+ve ion electrospray) m/z 228/229/231 (MH+), bromo-dichloropyridazine. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping