| 68% |
With N-Bromosuccinimide; dibenzoyl peroxide; In tetrachloromethane; for 2.5h;Heating / reflux; |
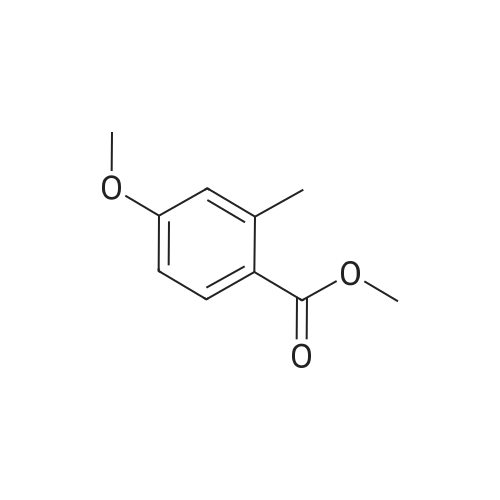
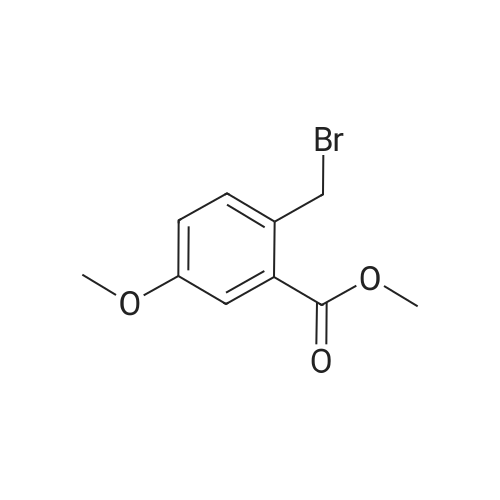
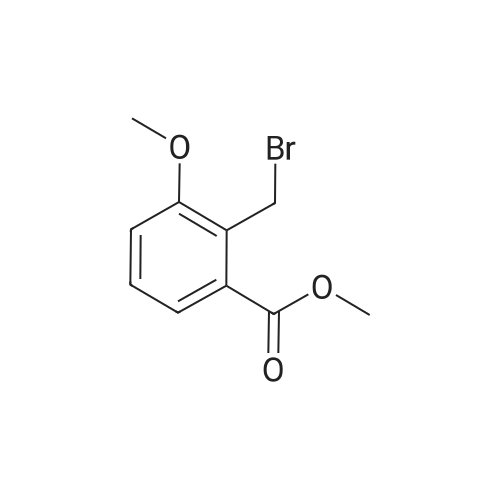
To a solution of <strong>[35598-05-1]4-methoxy-2-methyl-benzoic acid methyl ester</strong> (1.47 g, 8.16 mmol, 1.0 equiv; commercially available) in CCl4 (15 mL) was added N-bromosuccinimide (1.60 g, 8.97 mmol, 1.1 equiv) and dibenzoyl peroxide (0.198 g, 0.45 mmol, 0.05 equiv). The mixture was heated to reflux for 1.5 h, when TLC indicated that still some starling material was left. Therefore, additional N-bromosuccinimide (0.16 g, 0.90 mmol, 0.11 equiv) and dibenzoyl peroxide (0.080 g, 0.41 mmol, 0.18 equiv) was added and heating continued for 1 h. The reaction mixture was cooled down, poured on crashed ice, extracted with ethyl acetate, the combined organic phases washed with a sat. solution of NaCl, dried over Na2SO4 and concentrated by evaporation under reduced pressure. The crude material was purified with silica column chromatography eluting with hexane/ethyl acetate (95:5) affording 1.43 g (68%) of the title compound as yellow crystals. 1H NMR (300 MHz, CDCl3): delta 3.87 (s, 3H), 3.91 (s, 3H), 4.97 (s, 2H), 6.86 (dd, J=8.7 Hz, J=2.7 Hz, 1H), 6.97 (d, J=2.7 Hz, 1H), 7.99 (d, J=8.7 Hz, 1H). |
| 62% |
With N-Bromosuccinimide; dibenzoyl peroxide; In tetrachloromethane; for 18h;Heating / reflux; |
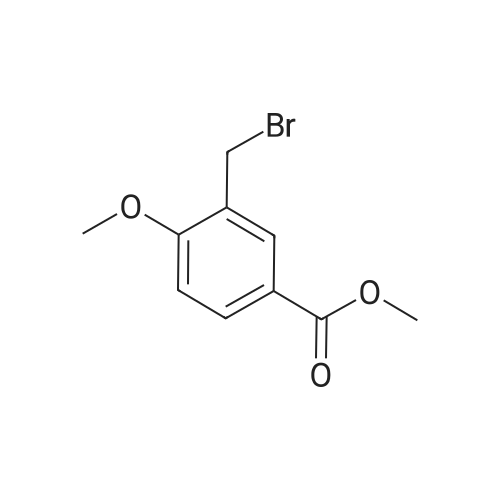
2-Bromomethyl-4-methoxy-benzoic acid methyl esterTo a solution of 4-Methoxy-2-methyl-benzoic acid methyl ester (6.92g, 38.4 mmol) in CCI4 (100 mL) was added N-bromosuccinimide (7.99 g, 1.17 mmol). Benzoyl peroxide (1.82 g, 7.54 mmol) was added to the slurry and the mixture was heated at reflux for 18 h. The reaction was cooled and the solid was filtered off. The filtrate was absorbed onto silica to perform chromatography (0-10 % ethyl acetate/hexanes) to yield 2-Bromomethyl-4-methoxy- benzoic acid methyl ester, (6.12 g, 62 %, CASNo. 15365-25-0). MS: ES M+1 : 258.9, M+2 260.9 (258.0, 260.0). |
| 60% |
With N-Bromosuccinimide; dibenzoyl peroxide; In tetrachloromethane; for 24h;Reflux; |
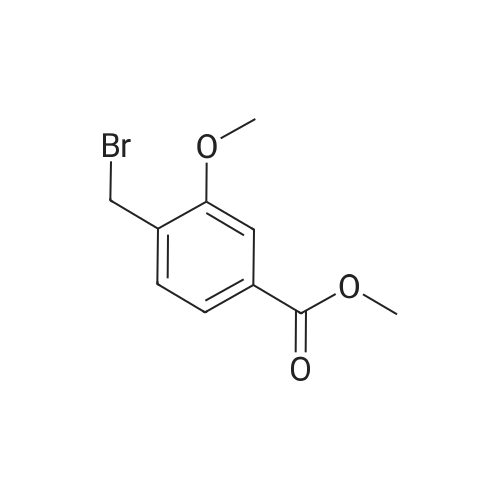
Methyl 2-methyl-4-(methyloxy)benzoate (1.1 g, 6.1 mmol), N-bromosuccinimide (1.2 g, 6.74 mmol), benzoyl peroxide (0.073 g, 0.30 mmol), and carbon tetrachloride (40 mL) were combined and the stirred reaction mixture was heated at reflux for 24 h under a nitrogen atmosphere. The reaction mixture was allowed to cool at room temperature and filtered through a pad of Celite. The pad was washed with ethyl acetate. The filtrate was concentrated to give the crude product which was purified by flash chromatography over silica with a hexanes:dichloromethane gradient (100:0 to 50:50) to give 0.95 g (60%) of methyl 2-(bromomethyl)-4-(methyloxy)benzoate as an oil which solidified to a white solid. 1U NMR (400 MHz, CDCl3): delta 7.98 (d, J = 9 Hz, IH), 6.96 (d, J = 3 Hz, IH), 6.85 (dd, J = 9, 3 Hz, IH), 4.96 (s, 2H), 3.90 (s, 3H), 3.86 (s, 3H). |
| 58% |
With N-Bromosuccinimide; dibenzoyl peroxide; In tetrachloromethane; for 4.5h;UV-irradiation; Heating / reflux; |
A mixture of <strong>[35598-05-1]4-methoxy-2-methyl-benzoic acid methyl ester</strong> (20 g, 111 mmol), N-bromo- succinimide (20.7 g, 117 mmol) and dibenzoylperoxide (0.54 g, 2 mmol) in [CC14] (150 mL) was irradiated with a 300 W lamp. The reaction maintains a steady reflux and after 4.5 h, the lamp was removed and the mixture cooled to [5 C.] The mixture was then filtered, the filtrate evaporated and the residue purified twice by chromatography [(SI02,] Heptane: Di- ethyl ether: 95: 5 to 85: 15) to afford the title product (16.6 g, 58%) as a white solid. MS m/e = 258.1 (M-H+). |
|
With N-Bromosuccinimide; dibenzoyl peroxide; In benzene; at 75℃; for 2h; |
Compound 9C (prepared according to the procedure described by Wyrick, S. D. et al. Journal of Medicinal Chemistry, 1987, 30(10), 1798-806) (3.33 g, 18.5 mmol) was dissolved in dry benzene (40 ml). NBS (3.45 g, 19.4 mmol) and benzoyl peroxide (134 mg, 0.55 mmol) were added. The solution was stirred in a 75 C oil bath for about 2 hours. After cooling down, the solid was filtered and washed with Et2O (150 mL). The organic solution was then washed with water (50 ml) twice, dried over Na2SO4 or MgSC>4, filtered, and concentrated by rotary evaporator. The crude product was dried under vacuum to give compound 9D which was used without further purification. 1H-NMR appeared to indicate that approximately 75% of this material was compound 9D. |
|
With N-Bromosuccinimide; acetic acid; In tetrachloromethane; at 90℃; for 3h; |
[0180] 4-Fluoro-2-methyl-benzoic acid methyl ester (600 mg, 3.6 mmol), N-bromosuccinimide (635 mg, 3.6 mmol) and benzoyl peroxide (43 mg, 1.79 mmol) in CCU (15 mL) was stirred at 90 C for 3 hours. The reaction was cooled to room temperature, filtered, washed with CCI4 and the solvent was removed under reduced pressure to provide a white solid. .H NMR (300 MHz, CDCI3): 5 8.01 (d, 1H), 6.99 (d, 1H), 6.88 (dd, 1H), 4.98 (s, 2H), 3.93 (s, 3H), 3.89 (s, 3H). [0181] The following compounds were made in a similar fashion: |
|
With N-Bromosuccinimide; dibenzoyl peroxide; In chloroform; at 65℃; for 5h;Inert atmosphere; Schlenk technique; Reflux; |
General procedure: To a solution of 6.0 g (0.04 mol) of methyl 2-methylbenzoate derivatives in 38 mL of chloroform, 7.5 g (0.042 mol) of N-bromosuccinimide and 0.078 g of benzoyl peroxide were added and carefully warmed up to 65 C until reaction started. Then the mixture was refluxed for 5 h. After cooling down to room temperature, the deposit of succinimide was filtered. The solvent was removed under reduced pressure and the crude product was used in the next step without further purification. To a solution of functionalized methyl 2-(bromomethyl)benzoate (6.55 mmol), substituted phenol (8.5 mmol), K3PO4 (16.4 mmol) and toluene 20 mL were added to Schlenk under argon. The resulting solution was stirred to 110 C for 5 h. The progress of the reaction was monitored by TLC. The mixture was extracted with EtOAc, washed with water, brine and the combined organic layers were dried over anhydrous Na2SO4and the solvent was removed under reduced pressure. The crude product was used in the next step without further purification. To the solution of the ester (0.015 mol) in MeOH (73 mL), was added 13 mL aqueous KOH (20%) and refluxed at 80C for 5 h. MeOH was removed and the aqueous phase was washed with DCM. After acidifying with HCl (10%) the deposit was collected and washed with water. |
|
With N-Bromosuccinimide; 2,2'-azobis(isobutyronitrile); In chloroform; for 2.5h;Reflux; |
To a solution of methyl o-methylbenzoate (1.00 equiv) in CHCl3 (2-5 mL per mmol of methyl o-methylbenzoate) or CCl4 (5 mL per mmol of methyl o-methylbenzoate) was added NBS(1.10-2.20 equiv) and AIBN (0.02-0.04 equiv). The reaction was refluxed for 2-22 h before being cooled to rt. The solvent was then removed in vacuo (CHCl3 solvent) or via distillation (CCl4 solvent) within a fumehood to afford the desired brominated compound. CAUTION: CHCl3 and especially CCl4 are extremely toxic and carcinogenic and thus must be handled cautiously with gloves and within a fumehood appropriately ventilated. |
|
With N-Bromosuccinimide; dibenzoyl peroxide; In 1,2-dichloro-ethane; at 80℃; for 12h; |
General procedure: A mixture of methyl 5-fluoro-2-methylbenzoate (0.5 g, 3.0 mmol),NBS (0.18 g, 3.0 mmol), and di-benzoyl peroxide (BPO) (36 mg, 0.15 mmol) in 1,2-dichloroethane (5 mL) was heated at 80 C for 12 huntil all starting material was consumed. The reaction was cooled to room temperature, and the precipitated solid was removed by filtration and washed with ethers (10 mL). The filtrate was concentrated in vacuo and the residue was partitioned between 2 N NaHCO3 (15 mL) and ethers (15 mL). The organic layer was separated, dried over MgSO4, filtered and concentrated to give a crude product (0.65 g, 89%), which was used in the next step reaction without further purification. |
|
With N-Bromosuccinimide; dibenzoyl peroxide; In 1,2-dichloro-ethane; at 80℃; for 12h; |
General procedure: A mixture of methyl 3-bromo-2-methylbenzoate (500 mg, 3.3 mmol), NBS (770.4 mg, 4.3 mmol), and di-benzoyl peroxide (BPO, 80.7 mg, 0.3 mmol) in 1,2-dichloroethane (10 mL) was heated at 80 C for 12 h. The reaction mixture was cooled to room temperature, and the precipitated solid was removed by filtration and washed with ethers (10 mL). The filtrate was concentrated in vacuo and the residue was partitioned between 2 N NaHCO3 (15 mL) and ethers (15 mL). The organic layer was separated, dried over NaSO4, filtered and concentrated to give a crude product (683.2 mg, 89.6%), which was used in the next step reaction without further purification. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping