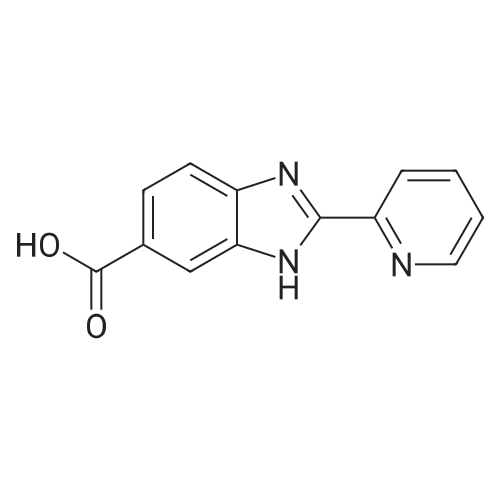
| 80% |
With water; sodium hydroxide; In methanol; at 80℃;Product distribution / selectivity; |
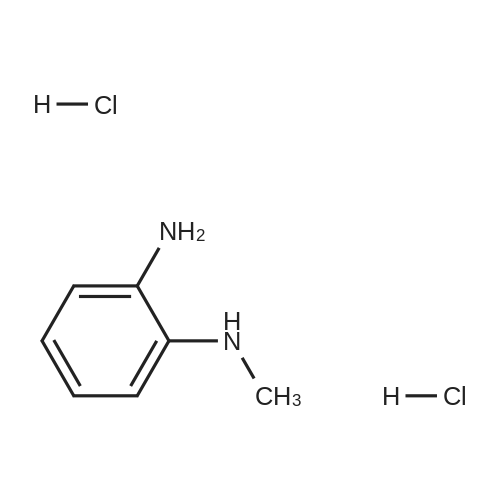
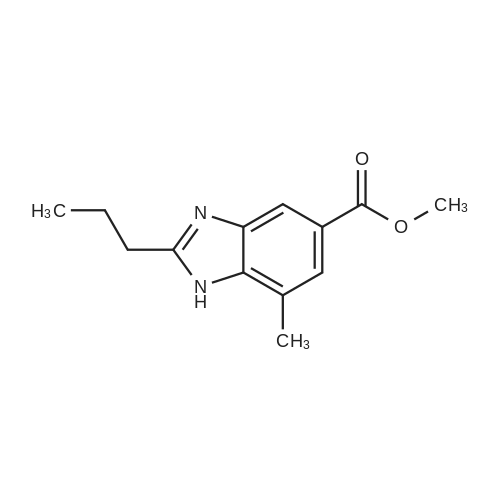
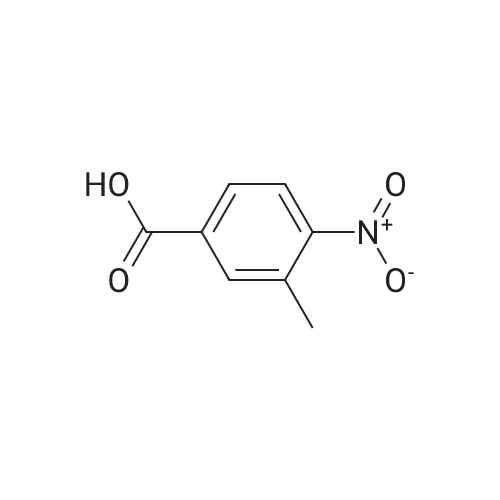
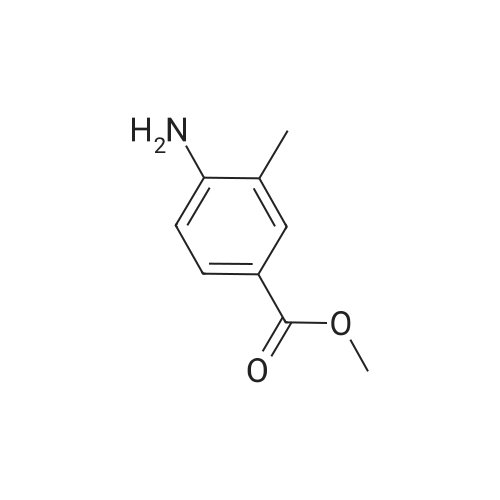
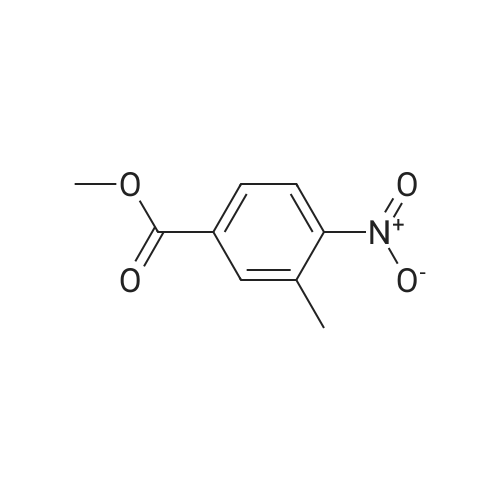
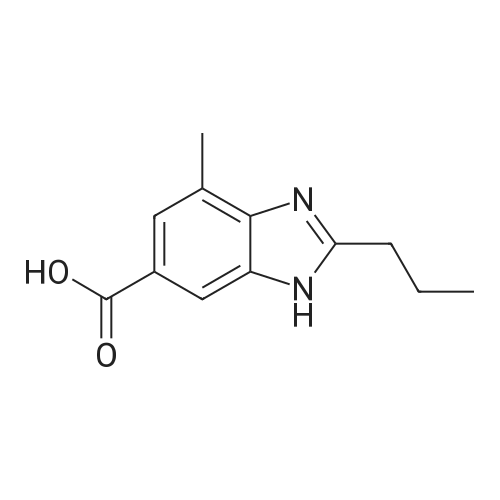
Example 1:Methyl-4-methyl-2-propyl-lH-benzimidazole-6-carboxylate (40 gms, 0.172 moles) was suspended in methanol (200 ml) and stirred for few minutes. Sodium hydroxide (13.76 gms, 0.344 moles) dissolved in water (50 ml) was added to the reaction mass and the temperature is maintained at 80C. After completion of the reaction, methanol was evaporated under reduced pressure and water (100 ml) was added to the crude mass at 5-10 C. Then adjusted the pH of the crude mass to 6- 6.5 and the obtained solid was filtered. The solid was washed with water and dried at temperature of 45-50 C under vacuum (700 mm) to yield 2-n-propyl-4-methyl- 6-carboxy benzimidazole (30gms, Yield: 80 %). |
|
|
Reference Example A3: 4-Methyl-6-(2-oxazolyl)-2-propylbenzimidazole [step 1] 6-Methoxycarbonyl-4-methyl-2-propylbenzimidazole (EP502314; 500 mg, 2.29 mmol) was suspended in ethanol (15 mL), 4 mol/L aqueous sodium hydroxide solution (3.1 mL) was added, and the mixture was stirred under reflux for 7 hr. The mixture was concentrated under reduced pressure, and water (20 mL) was added. Under ice-cooling, the mixture was adjusted to pH 1 with 2 mol/L hydrochloric acid, and extracted with chloroform. The organic layer was washed with brine, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The residue was dissolved in dichloromethane, aminoacetaldehyde dimethylacetal (0.50 mL, 4.58 mmol), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (527 mg, 2.75 mmol) and 1-hydroxybenzotriazole (421 mg, 2.75 mmol) were added, and the mixture was stirred at room temperature for 6 hr. To the mixture were added saturated aqueous sodium hydrogen carbonate solution (100 mL) and chloroform (100 mL), and the organic layer was washed with brine, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure to give 6-(2,2-dimethoxyethylcarbamoyl)-4-methyl-2-propylbenzimidazole. ESI-MS m/z; 306 (M + H)+; 1H-NMR (CDCl3, delta): 1.02 (t, J = 7.3 Hz, 3H), 1.82-1.97 (m, 2H), 2.65 (s, 3H), 2.92 (t, J= 7.3 Hz, 2H), 3.45 (s, 6H), 3.64 (t, J = 5.3 Hz, 2H), 4.52 (t, J = 5.3 Hz, 1H), 6.42 (s, 1H), 7.89 (s, 1H). |
|
With sodium hydroxide; In methanol; water; for 2h;Reflux; |
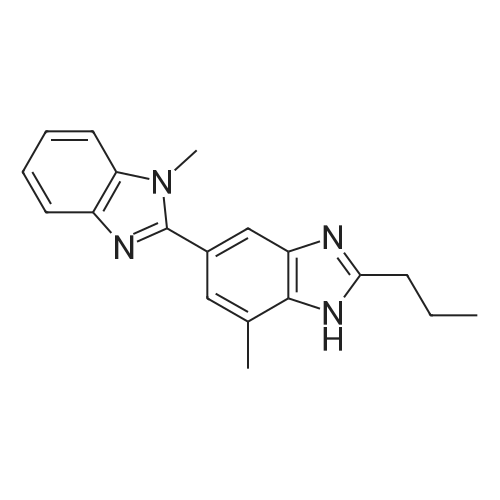
General procedure: A solution of an appropriate ester (25.01mmol) in methanol (25mL) was added to a solution of NaOH (2.0g) in water (25mL), and the mixture was heated under reflux for 2h. After evaporation of methanol, the pH was adjusted to 4-5 by addition of aqueous citric acid. The precipitated solid was filtered, washed with ethanol and dried to yield carboxylic acid. The resulting compound was dissolved in polyphosphoric acid (10mL) at 150C. N-Methyl-o-phenylenediamine dihydrochloride (3.65g, 18.8mmol) was added to the mixture for 4 times in 4h. After stirring at 150C for 10h, the mixture was cooled and then poured into ice water (30mL). The pH was adjusted to 10 by addition of concentrated ammonia (ice cooling). The precipitated solid was filtered off, dried, and boiled in ethyl acetate (300mL). After cooling, the precipitated solid was filtered off, washed with diethyl ether, and dried to give the product as white solid. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping