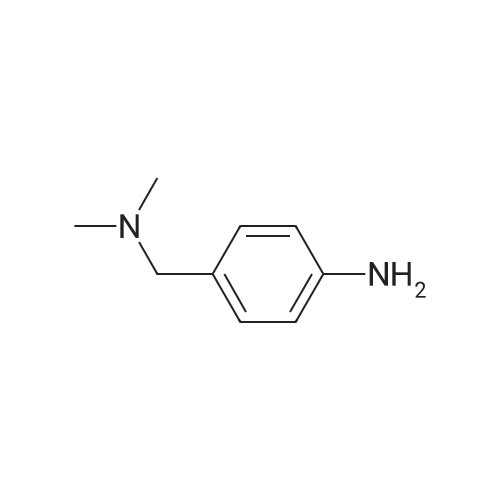
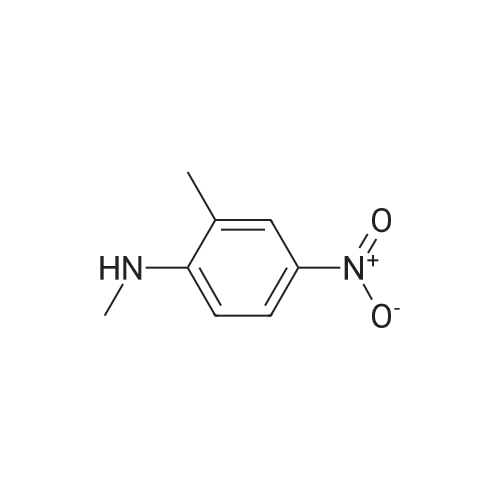
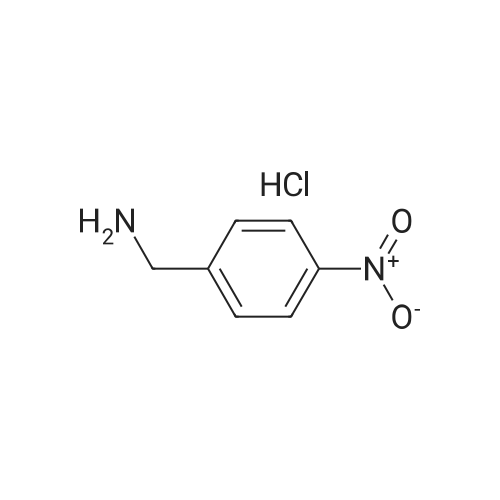
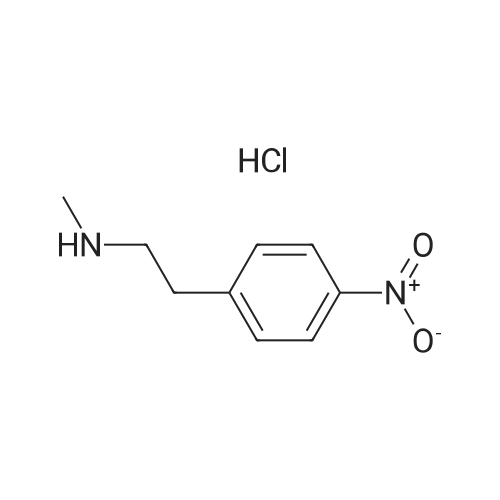
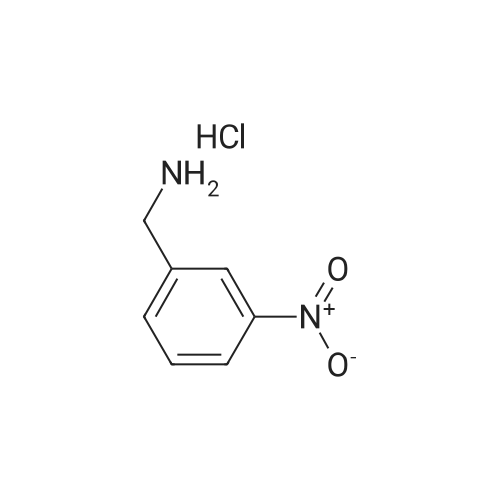
| 78.2% |
With iron(III) chloride; hydrazine hydrate; In ethanol; at 60 - 70℃; for 5h; |
General procedure: 1 (0.05 mol) was added to 90% ethanol (100 mL),The mixture was heated to 60 C with stirring, and ferric chloride (0.001 mol) and activated carbon (0.015 mol) were added. At 70 C, hydrazine hydrate (0.5 mol) was added dropwise, and the mixture was refluxed for 5 hours. After completion of the reaction, the mixture was filtered under suction, and the filtrate was concentrated. Water (200 mL), dichloromethane (100 mL×3)Concentrated to a colorless liquid orWhite solid 2a-c. |
| 75% |
With hydrazine hydrate; iron(III) chloride hexahydrate; pyrographite; In ethanol; at 65 - 70℃; for 5h; |
A mixture of 3.5 g (19.4 mmol) of compound (b) in ethanol was heated to 65C. 0.83 g (0.39 mmol) of FeCl36H2O and 0.07 g (5.85 mmol) of activated carbon were added and 9.8 g (194 mmol) of 80% hydrazine hydrate was added dropwise at a rate maintaining the temperature below 70C .The reaction was heated at reflux for 5 hours, cooled to room temperature and concentrated. 500 ml of water was added and the reaction solution was extracted three times with DCM.The organic extracts were mixed, dried over sodium sulfate, and concentrated to obtain 3.2 g (75%) of a colorless cucumber compound (c). |
|
palladium; In ethanol; |
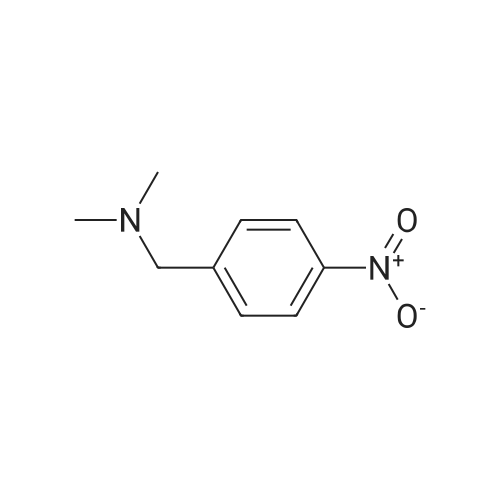
REFERENCE EXAMPLE 15 In ethanol (100 ml) was dissolved dimethyl-4-nitrobenzylamine (20.7 g), and to the mixture was added dried 10% palladium on carbon (1.00 g). Under hydrogen atmosphere, the mixture was stirred at room temperature under atmospheric pressure for 20 hours. The palladium was filtered off, and the filtrate was concentrated. The residue was separated and purified with column chromatography (ethyl acetate) to give 4-aminobenzyl-dimethylamine (8.75 g) as pale yellow oil. 1 H NMR (200 MHz, CDCl3) delta: 2.21 (6H, s), 3.31 (2H, s), 3.53-3.70 (2H, br), 6.65 (2H, d, J=8.4 Hz), 7.08 (2H, d, J=8.4 Hz). |
|
With hydrogen;palladium 10% on activated carbon; In methanol; under 3750.38 Torr; for 16h; |
6.9 g (38.1 mmol) 4-nitro-dimethylbenzylamine are dissolved in 100 mL MeOH, combined with 1 g of palladium on activated charcoal (10 % Pd) and stirred for 16 h under a hydrogen atmosphere (5 bar) in the autoclave. After this time, total conversion can be detected (monitoring of reaction by HPLC). The catalyst is filtered off and all the volatile constituents are eliminated in vacuo. 6.8 g crude C-I are obtained, which is used without further purification in the syntheses of Examples 1, 2 and 6. MS-ESI+: 151 (M+H)+ |
|
|
Stannous chloride (28.1 g, 148 mmol) was added to a solution of Compound 4a2 (5.33 g, 29.6 mmol) in ethanol (200 mL) and heated to 60 C. Sodium borohydride (0.560 g, 14.8 mmol) in ethanol (80 mL) was added dropwise. Two hrs later the reaction mixture was added to ice water. The slurry was filtered through Celite 545 and the filtrate cake rinsed with ether. The collected liquors were brought to pH 11 with 1N NaOH and extracted with ether. The combined organic layers were washed with water, dried over MgSO4 and filtered. The dried organic solution was then reduced in vacuo to provide 4-dimethylaminomethyl-phenylamine Compound 4a which was used in the next step without further purification. MS 207 (MH+). |
|
With hydrogenchloride; iron; acetic acid; In ethanol; water; for 0.75h;Reflux; |
PREPARATION 6: 4-((DIMETHYLAMINO)METHYL)ANILINE To a reaction flask was added N,N-dimethyl(4-nitrophenyl)methyamine (4.553 g). Anhydrous ethanol was added to dissolve N,N-dimethyl(4-nitrophenyl)methyamine. After adding acetic acid (5.2 mL), the mixture was mechanically stirred. To 1 N HCl (40 mL) was added iron powders (11.339g). The iron powders were immersed for 10 min and filtered by suction. The filter cake was washed with absolute ethanol and then added into the reaction flask. The mixture was warmed in an oil bath to reflux, and maintained under reflux until the reaction was completed (about 35 min). After the reaction was completed, the reaction solution was filtered by suction. The filter cake was washed with absolute ethanol. The filtrate was concentrated and then added into distilled water (100 mL). The pH of the mixture was adjusted with NaOH to 14. Lots of solids were precipitated. The solids were filtered by suction. The filtrate was extracted with dichloromethane (100 mL * 1), and the aqueous phase was discarded. To the organic phase was added distilled water (60 mL). The pH was adjusted with 2 N HCl to 2. The resultant product was mixed and stood to separate. The organic phase was discarded. The pH of the aqueous phase was adjusted with NaOH to 7. The mixture was extracted with dichloromethane (40 mL * 2) and the aqueous phase was discarded. The organic phase was washed with distilled water (40 mL * 1) once and the aqueous phase was discarded. The organic phase was directly concentrated to give the target compound. |
|
With hydrogenchloride; iron; acetic acid; In ethanol; water; for 0.583333h;Reflux; |
To a reaction flask was added N,N-dimethyl(4-nitrophenyl)methyamine (4.553 g). Anhydrous ethanol was added to dissolve N,N-dimethyl(4-nitrophenyl)methyamine. After adding acetic acid (5.2 mL), the mixture was mechanically stirred. To 1 N HCl (40 mL) was added iron powders (11.339 g). The iron powders were immersed for 10 min and filtered by suction. The filter cake was washed with absolute ethanol and then added into the reaction flask. The mixture was warmed in an oil bath to reflux, and maintained under reflux until the reaction was completed (about 35 min). After the reaction was completed, the reaction solution was filtered by suction. The filter cake was washed with absolute ethanol. The filtrate was concentrated and then added into distilled water (100 mL). The pH of the mixture was adjusted with NaOH to 14. Lots of solids were precipitated. The solids were filtered by suction. The filtrate was extracted with dichloromethane (100 mL*1), and the aqueous phase was discarded. To the organic phase was added distilled water (60 mL). The pH was adjusted with 2 N HCl to 2. The resultant product was mixed and stood to separate. The organic phase was discarded. The pH of the aqueous phase was adjusted with NaOH to 7. The mixture was extracted with dichloromethane (40 mL*2) and the aqueous phase was discarded. The organic phase was washed with distilled water (40 mL*1) once and the aqueous phase was discarded. The organic phase was directly concentrated to give the target compound. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping