| 55% |
|
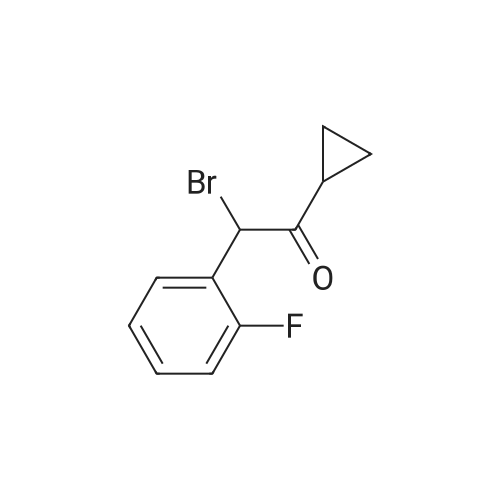
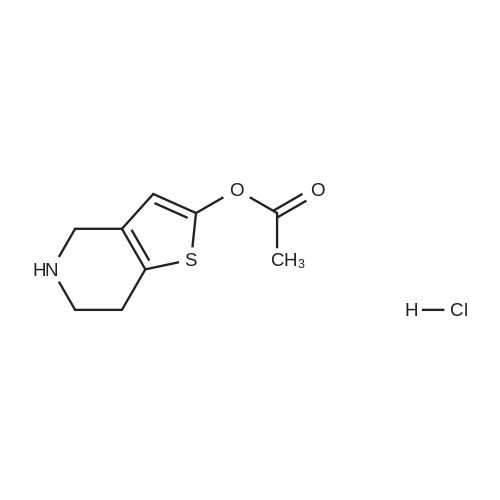
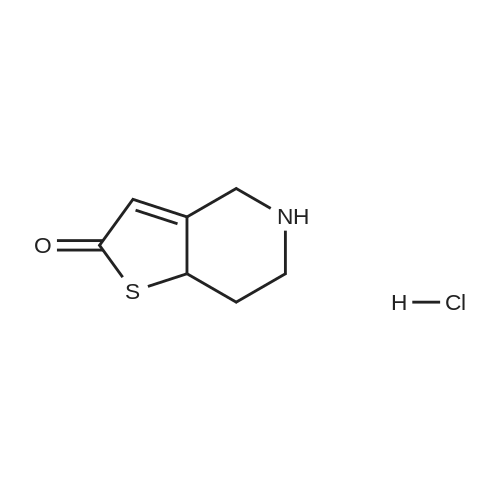
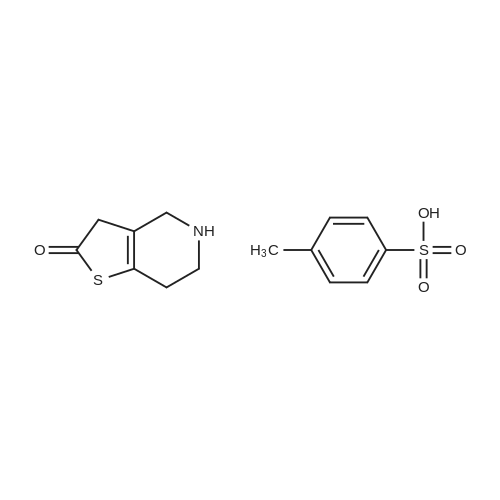
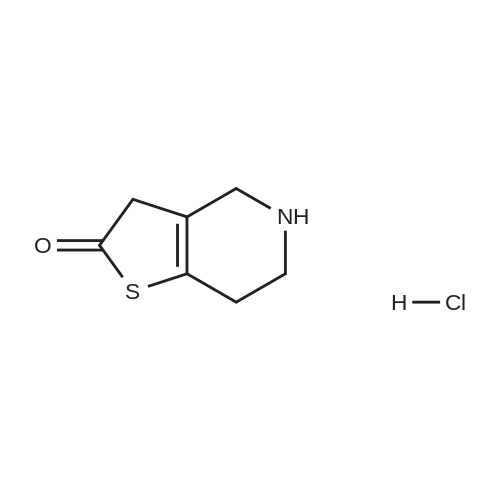
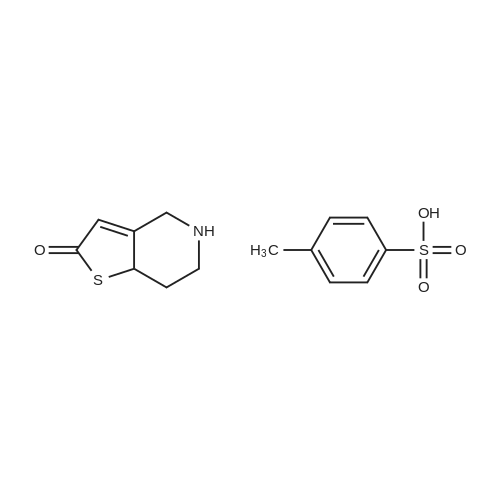
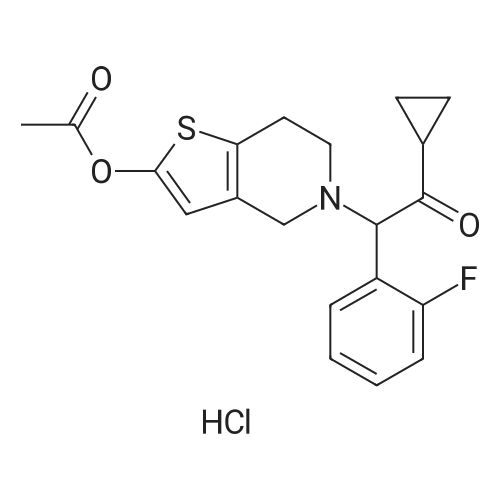
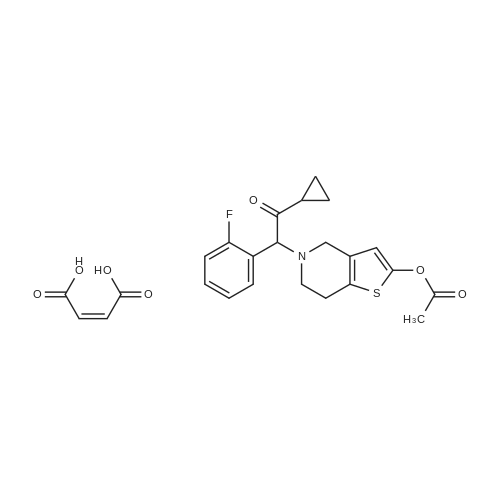
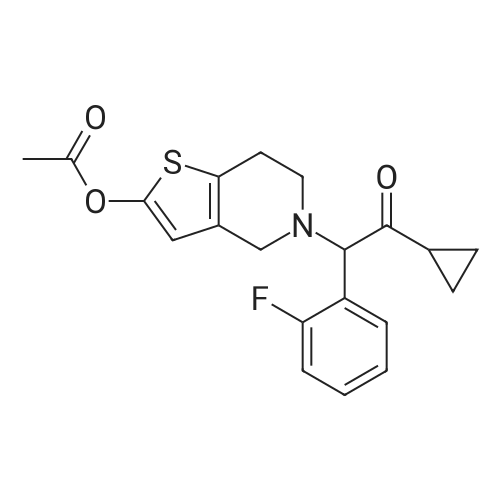
Example 82-Acetoxy-5-(2-fluoro-a-cyclopropyl-carbonyI-benzyl)-4,5,6,7- tetrahydro-4H-thieno[3,2-c] pyridine (prasugrel, compound of theFormula (I))65.5 g (0.2 mol) of 5,6,7,7a-tetrahydro-4H-thieno[3,2-c]-pyridine-2- one joara-toluenesulfonate (compound of the Formul (II), HA=PTSA) and 160 ml of N,N-dimethylformamide are combined. To this mixture are added 75.3 cm (56.9 g; 0.44 mol) NN-diisopropylethylamine (DIPEA). Subsequently while cooling the reaction mixture on an ice/water bath, 53.8 g of 2-bromo-l-cyclopropyl-2-(2-fmorophenyl)- ethanone of the Formula (III) having 95.5% content according to gas chromatographic assay, dissolved in 94 ml (88.7 g) of Nu,Nu- dimethylformamide are added dropwise in 30 minutes and the thus obtained mixture is stirred for one hour at room temperature. Thereafter 37.65 cm3 (28.43 g; 0.22 mol) of DIPEA are added to the reaction mixture and while stirring intensively and maintaining the temperature between 15 and 20 C, 28.4 ml (30.6 g; 0.30 mol) of acetic anhydride are added dropwise. Addition of acetic anhydride is followed by stirring for one hour at room temperature. The reaction mixture is poured into a mixture of ice, water and ethylacetate. The layers are separated, the aqueous layer is washed with ethylacetate.The organic layer and the washings are combined and dried over magnesium sulfate. The solution is evaporated in vacuo, and ethanol is added to the residue. The mixture is cooled to a temperature between 0 and 5 C, the crystals are filtered and washed with ethanol.Thus 44.7 g (60.0 %) of raw prasugrel are obtained, which are recrystallized from ethanol.Yield, 41.1 g (55.0 %) colourless, crystalline solid.Assay (measured by high-performance liquid chromatography), better than 99.80 %.The individual concentration of the impurities of the Formulae(XXIV) and (XXIVa) is less than 25 ppm each.Total yield (calculated for 4,5,6,7-tetrahydro-thieno[3,2-c]pyridine hydrochloride of the Formula (IX)), 45.7 %.Melting point, 120-121 CIR (KBr, cm-1): 3388, 2920, 2767, 1758, 1704, 1586, 1488, 1369, 1217, 1194, 1127, 1011.1H-NMR (CDC13, 500 MHz): 7,47 (1H, td, J=7,5; 1,8 Hz); 7,30 (1H, m); 7,16 (1H, td, J=7,5; 1,1 Hz); 7,10 (1H, td, J=8,2; 1,1 Hz); 6,26 (1H, s); 4,82 (1H, s); 3,56 (1H, d, J=14,3 Hz); 3,48 (1H, d, J-14,3 Hz); 2,90 (1H, m); 2,78 (3H, m); 2,28 (1H, m); 2,23 (3H, s); 1,05 (1H, m); 1,00 (1H, m); 0,84 (2H, m).13C-NMR (CDCI3, 125 MHz): 207,4; 167,5; 161,1; 149,4; 130,4; 129,7; 129,3; 125,6; 124,2; 122,0; 115,6; 112,8; 71,5; 50,3; 48,3, 24,9; 20,4; 18,1, 11,8, 1 1,3. Elemental analysis calculated for the Formula C20H20FNO3S (M: 373,45)Calculated: C 64.33; H 5.40; N 3.75; S 8.59 %.Measured: C 64.18; H 5.50; N 3.69; S 8.75 %. |
| 55% |
|
Example 4 Preparation of 2-Acetoxi-5-(2-fluor-alpha-cyclopropyl-carbonyl-benzyl)-4,5,6,7-tetrahydro-4H-tieno[3,2-c]pyridine (prasugrel, I) 160 cm3 of DMF are added to 65.5 g (0.2 mol) of 5,6,7,7a-tetrahydro-4H-tieno[3,2-c]-pyridine-2-on para-toluenesulfonate (II, HA=PTSA). 75.3 cm3 (56.9 g; 0.44 mol) of N,N-diisopropyl-ethyl-amine (DIPEA) are added to the solution and 55.4 g of 2-bromo-1-cyclopropyl-2-(2-fluorophenyl)-ethanon (III) (containing 92.8% of GC) dissolved is 94 cm3 (88.7 g) of dimethyl-formamide is added dropwise within app. 30 minutes under ice water cooling. The mixture is stirred for 1 hour at room temperature. 37.65 cm3 (28.43 g; 0.22 mol) of DIPEA are added to the reaction mixture and under intensive stirring 28.4 cm3 (30.6 g; 0.30 mol) of acetic acid anhydride are added dropwise. The mixture is stirred for 1 hour at room temperature. The reaction mixture is poured onto the mixture of ice water and ethylacetate. The phases are separated and the aqueous phase is extracted with ethylacetate. The collected organic phases are dried on MgSO4. The solvent is removed in vacuo and ethanol is added to the remaining product. After cooling to 0-5 C. the precipitated crystals are filtered, washed with ethanol. The yield is 44.7 g (60.0%) crude prasugrel base.Yield: 41.1 g (55.0%) colorless, crystalline product, HPLC purity >99.80%.[0068]Yield for the whole synthetic process, calculated on the 4,5,6,7-tetrahydro-tieno[3,2-c]pyridine hydrochloride of the formula (VII) is 45.7%.[0069]Mp.: 120-121 C.[0070]IR (KBr, cm-1): 3388, 2920, 2767, 1758, 1704, 1586, 1488, 1369, 1217, 1194, 1127, 1011.[0071]1H-NMR (CDCl3, 500 MHz): 7.47 (1H, td, J=7.5; 1.8 Hz); 7.30 (1H, m); 7.16 (1H, td, J=7.5; 1.1 Hz); 7.10 (1H, td, J=8.2; 1.1 Hz); 6.26 (1H, s); 4.82 (1H, s); 3.56 (1H, d, J=14.3 Hz); 3.48 (1H, d, J=14.3 Hz); 2.90 (1H, m); 2.78 (3H, m); 2.28 (1H, m); 2.23 (3H, s); 1.05 (1H, m); 1.00 (1H, m); 0.84 (2H, m).[0072]13C-NMR (CDCl3, 125 MHz): 207.4; 167.5; 161.1; 149.4; 130.4; 129.7; 129.3; 125.6; 124.2; 122.0; 115.6; 112.8; 71.5; 50.3; 48.3, 24.9; 20.4; 18.1, 11.8, 11.3.[0073]Elementary analysis [calculated on the basis of the formula of C20H20FNO3S (M: 373.45)][0074]Calculated: C 64.33; H 5.40; N 3.75; S 8.59.[0075]Measured: C 64.18; H 5.50; N 3.69; S 8.75. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping