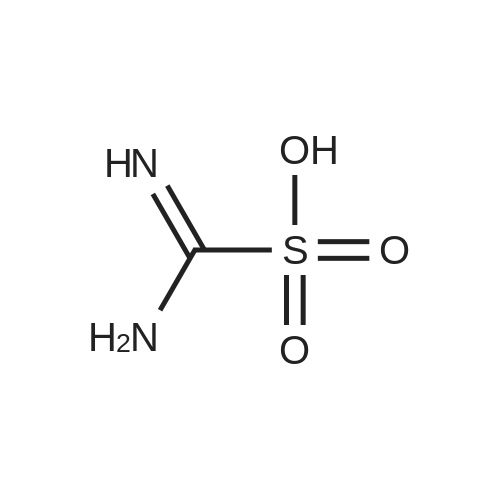
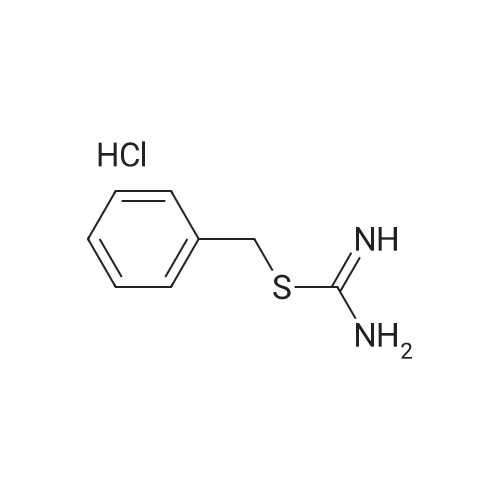
| 51% |
With isopentyl nitrite; In acetonitrile; at 60℃; for 2h; |
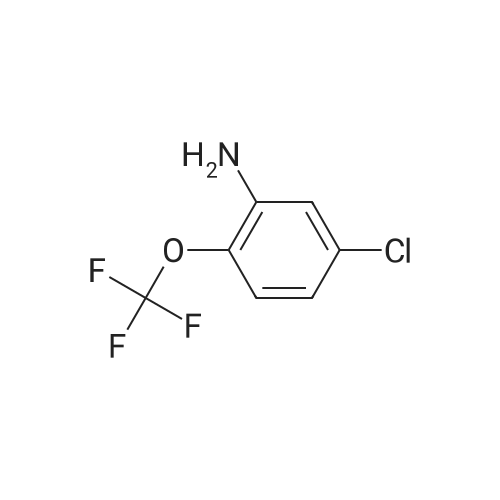
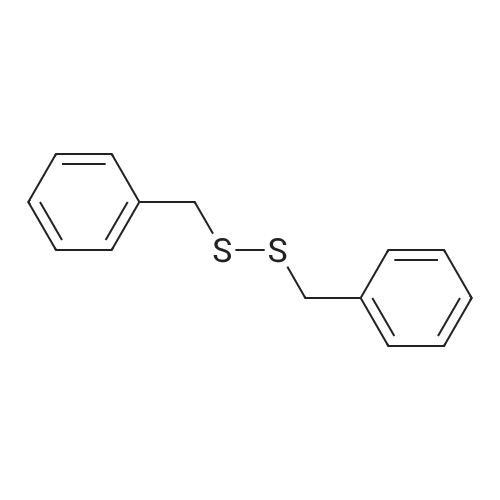
To a suspension of 5-chloro-2- ( trifluorometnyl ) aniline (5.00 g, 23.6 mmol) and dibenzyl disulfide (4.66g, 18.9 mmol) in acetonitrile (75 mL) , Isoamyl nitrite (3.46 mL, 26.0 mmol) was slowly added at 60C in an oil bath, and the mixture was stirred at the same temperature as above for 2 hours. The reaction mixture was cooled and then concentrated under reduced pressure, and the residue was purified in an automatic chromatography apparatus (n-hexane/ethyl acetate = 100/0 - 95/5) to prepare 2- (benzylsulfanyl ) -4-chlorophenyl trifluoromethyl ether (3.86 g, yield: 51%) . To a mixture of the obtained 2- (benzylsulfanyl ) -4-chlorophenyl trifluoromethyl ether (4.84 g, 15.2 mmol), acetic acid(4.5 mL) and water (3 mL) in acetonitrile (120 mL) , 1, 3-dichloro-5, 5-dimethylhydantoin (5.98 g, 30.4 mmol) was added under ice cooling, and the mixture was stirred at the same temperature as above for 3 hours. The mixture was diluted by addition of a saturated aqueous solution of sodium bicarbonate, extracted with ethyl acetate. The organic layer was washed with a saturated aqueous solution of sodium chloride and dried over anhydrous sodium sulfate. After filtration, the solvent was distilled off under reduced pressure, and the residue was purified in an automatic chromatography apparatus(hexane/ethyl acetate = 100/0 - 85/15) to obtain the title compound (3.64 g, yield: 81%) . NMR spectrum (CDC13, 400MHz) delta: 8.09 (1H, d, J =2.3 Hz), 7.75 (1H, dd, J = 9.0, 2.7 Hz), 7.50-7.47 (1H, m) . |
| 51% |
With isopentyl nitrite; In acetonitrile; at 20 - 60℃; for 2h; |
To a suspension of 5-chloro-2- (trifluoromethoxy)aniline (5.00 g, 23.6 mmol) and dibenzyl disulfide (4.66g, 18.9 mmol) in acetonitrile (75 mL), isoamyl nitrite (3.46 mL, 26.0 mmol) was slowly added at 60C in an oil bath, and the mixture was stirred at the same temperature as above for 2 hours. The reaction mixture was cooled and then concentrated under reduced pressure, and the residue was purified in an automatic chromatography apparatus (n-hexane/ethyl acetate = 100/0 - 95/5) to prepare 2-(benzylsulfanyl)-4- chlorophenyl trifluoromethyl ether (3.86 g, yield: 51%) To a mixture of 2-(benzylsulfanyl)-4-chlorophenyl trifluoromethyl ether (4.84 g, 15.2 mmol) obtained in the above step, acetic acid (4.5 mL) and water (3 mL) in acetonitrile (l2OmL), 1,3-dichloro-5,5-dimethylhydantoin (5.98 g, 30.4 mmol) was added under ice cooling, and the mixture was stirred at the same temperature as above for 3 hours. The mixture was diluted by addition of a saturated aqueous solution of sodium bicarbonate, extracted with ethyl acetate. The organic layer was washed with a saturated aqueous solution of sodium chloride and dried over anhydrous sodium sulfate. After filtration, the solvent was distilled off under reduced pressure, and the residue was purified in an automatic chromatography apparatus (hexane/ethyl acetate = 100/0 - 85/15) to obtain the title compound (3.64 g, yield: 81%) ?H NNR spectrum (CDC13, 400MHz) oe: 8.09 (1H, d, J =2.3 Hz), 7.75 (1H, dd, J = 9.0, 2.7 Hz), 7.50-7.47 (1H, m) |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping