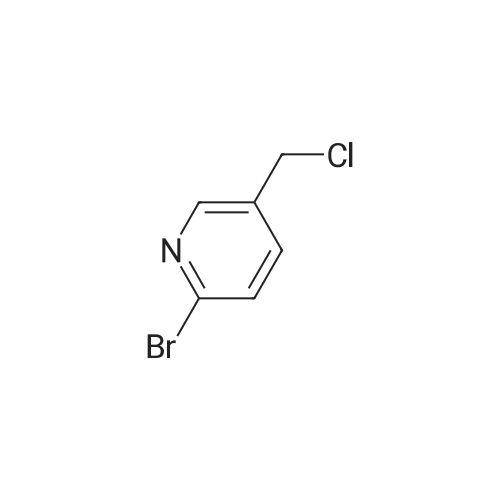
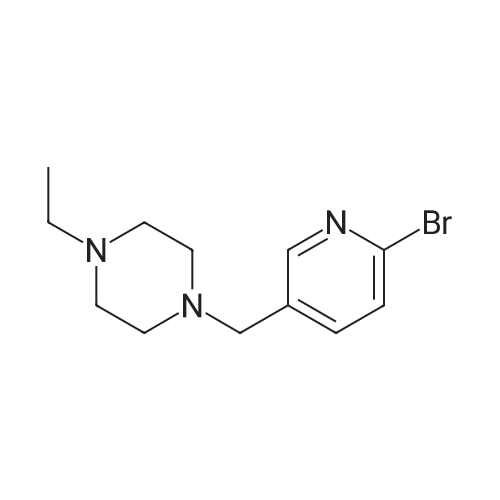
| 71% |
With sodium tris(acetoxy)borohydride In dichloromethane at 20℃; |
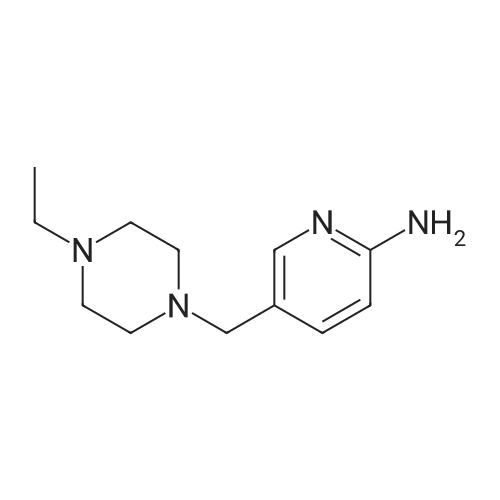
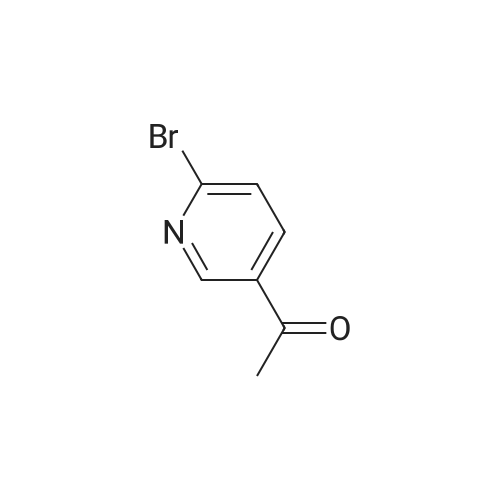
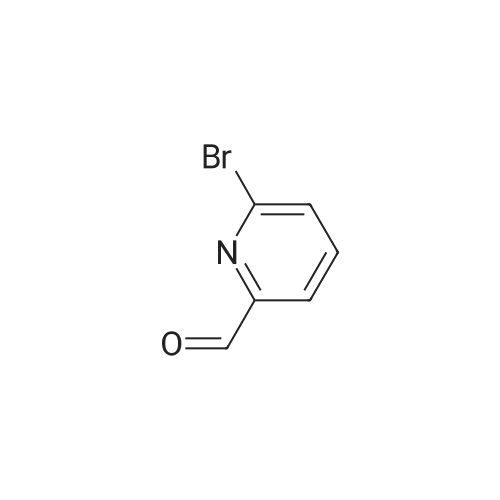
NaHB(OAc)3 (2.60g, 12.23mmol) was added to a solution of 137 6-bromonicotinaldehyde (1.50g, 8.15mmol) and 92 1-ethylpiperazine (0.90g, 8.15mmol) in 103 DCM (15mL). The mixture was allowed to stir at RT overnight, after which it was filtered and concentrated under a vacuum, and purified by silica gel column chromatography (DCM/MeOH=100:1–10:1) to obtain 138 1-((6-bromopyridin-3-yl)methyl)-4-ethylpiperazine (1.65g; yield, 71percent) as a yellow oil. Lithium bis(trimethylsilyl)amide (1N) (20mL, 20.0mmol) under N2 was added to a solution of 1-((6-bromopyridin-3-yl)methyl)-4-ethylpiperazine (2.80g, 10.0mmol), Cy-John-Phos (700.0mg, 2.0mmol), and Pd2(dba)3 (915.0mg, 1.0mmol) in dry toluene (30mL). Then the mixture was heated to 80°C overnight, cooled to RT, filtered and concentrated under a vacuum, and purified by silica gel column chromatography (DCM/MeOH=100:1–10:1) to give INT-7 (1.52g; yield, 69percent) as a brown solid. ESI-MS: m/z 221.2 [M+H]+. |
| 46% |
Stage #1: at 20℃; for 0.5 h;
Stage #2: With sodium tris(acetoxy)borohydride In dichloromethane at 20℃; for 12 h; |
To a solution of compound 5 (10 g, 54 mmol, 1 eq) in DCM (170 mL) was added compound 6 (7.4 g, 65 mmol, 1.2 eq). The mixture was stirred at rt for 30 minutes, then NaBH(OAc)3 (17.1 g, 81 mmol, 1.5 eq) was added portion wise. The mixture was stirred at rt for 12 h. After completion, the mixture was diluted with DCM (300 mL) and 2N NaOH (100 mL). The organic layer was separated and the aqueous layer was extracted with DCM (100 mL). The combined organic layers were dried over sodium sulfate, concentrated and purified by silica column chromatography to give the desired product (7 g, 46percent). NMR (300 MHz, CDCb) δ 8.30 (s, 1 H), 7.55 (d, J= 8.1 Hz, 1 H), 7.44 (d, J= 8.1 Hz, 1 H), 3.49 (s, 2 H), 2.55- 2.48 (m, 10 H), 1.12 (t, J= 7.2 Hz, 3 H). LCMS: (M+H)+: 283.9. |
| 11.5 kg |
With sodium tris(acetoxy)borohydride In dichloromethane at 20 - 30℃; for 12 h; Large scale |
Add neat 1-ethylpiperazine (5.6 kg) to a mixture of 6-bromo-pyridine-3- carbaldehyde (8.3 kg) and dichloromethane (186 kg). Then, add sodium (0086) triacetoxyborohydride (10.9 kg) in portions and stir at 20-30 °C for 12 hours. Quench the reaction into a mixture of dichloromethane (36 kg) and aqueous solution of sodium hydroxide 2 N (46 kg). Separate the layers and extract twice the aqueous layer with dichloromethane (24 X 2 kg). Combine the organic layers, wash with brine (50 X 2 kg) and remove the solvent under vacuum to afford 11.5 kg of the title compound. MS (ES+): m/z= 285 (M+H)+. |
| 1.48 g |
With formic acid; trimethyl orthoformate In acetonitrile for 4 h; Reflux; Inert atmosphere |
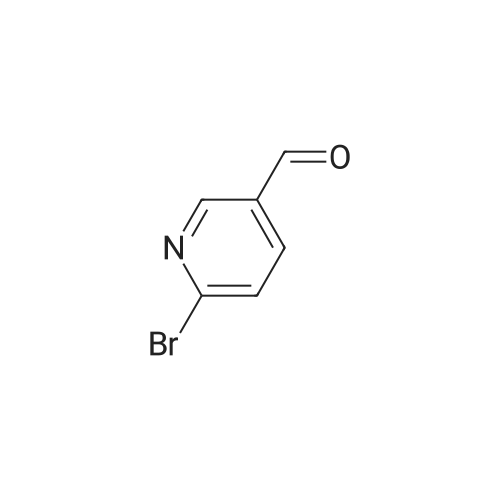
N-ethylpiperazine (1.59 g, 13.95 mmol), 2-bromo-5-pyridinecarbaldehyde (3.14 g, 16.88 mmol) were added successively to acetonitrile,To a solution of formic acid (2.10 mL, 55.80 mmol)Trimethyl orthoformate (3.07 mL, 27.90 mmol).Heating under reflux under nitrogen,After 4 hours the reaction solution was cooled to room temperature,Add 30 mL of water,15 mL of ethyl acetate,Liquid separation.The organic layer is the remaining 2-bromo-5-pyridinecarboxaldehyde.The aqueous layer was added with saturated sodium hydroxide to adjust the pH to 10,30 mL of ethyl acetate was added,Liquid separation,The aqueous layer was again added with 30 mL of ethyl acetate,Combined organic layer,Dried over anhydrous sodium sulfate and concentrated under reduced pressure to give the crude product.The product of formula XII-1 (1.48 g) was purified by column chromatography,As a colorless liquid. |
| 1.48 g |
With formic acid; trimethyl orthoformate In acetonitrile for 4 h; Reflux; Inert atmosphere |
N-Ethylpiperazine (1.59 g, 13.95 mmol) and 2-bromo-5-pyridinecarbaldehyde (3.14 g, 16.88 mmol) were sequentially added to acetonitrile and formic acid (2.10 mL, 55.80 mmol) Ester (3.07 mL, 27.90 mmol).The mixture was heated to reflux under nitrogen and the reaction was cooled to room temperature after 4h. 30mL of water and 15mL of ethyl acetate were added to separate the layers.The organic layer is the remaining 2-bromo-5-pyridinecarboxaldehyde.The aqueous layer was added with saturated sodium hydroxide to adjust the pH to 10, added with 30 mL of ethyl acetate, separated and the aqueous layer was added with 30 mL of ethyl acetate again. The combined organic layers were dried over anhydrous sodium sulfate and concentrated under reduced pressure to give the crude product.Purification by column chromatography gave 1.48 g of a colorless liquid.1H-NMR (300 MHz, CDCl 33)?: 8.29 d, J = 1.74Hz), 7.53-7.55 (1H, dd, J = 1.98Hz, 8.10Hz), 7.43 (1H, d, J = 8.10Hz), 3.47 (2H, s) , 2.38-2.48 (10H, m), 1.08 (3H, t, J = 7.14 Hz).HRMS (ESI): m / z 283.0684 |
| 1.64 g |
With sodium tris(acetoxy)borohydride In dichloromethane at 20℃; |
2-bromo-5-formylpyridine (1.5 g, 8.15 mmol), 1-ethylpiperazine (0.93 g, 8.15 mmol), and
dichloromethane (15 mL) were added to the reaction flask, and then NaHB(OAc)3 (2.58 g, 12.23 mmol) was added in batches.
Reaction was carried out at room temperature overnight.
The reaction product was filtered, concentrated and separated by column chromatography (DCM/MeOH = 100:1 to 10:1) to obtain the titled product (1.64g, yellow oil).
MS (ESI): mass calcd. for C12H18BrN3 285.1, m/z found 286.1 [M+H]+. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping