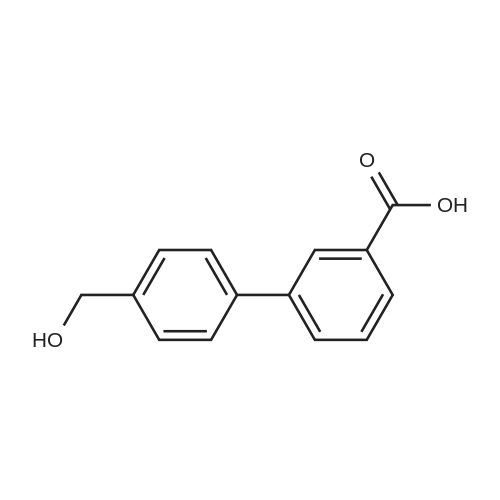
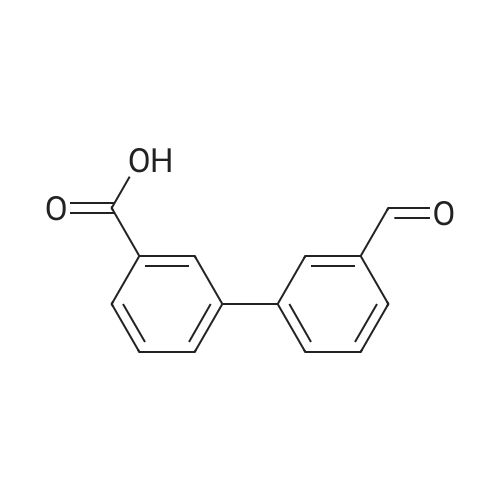
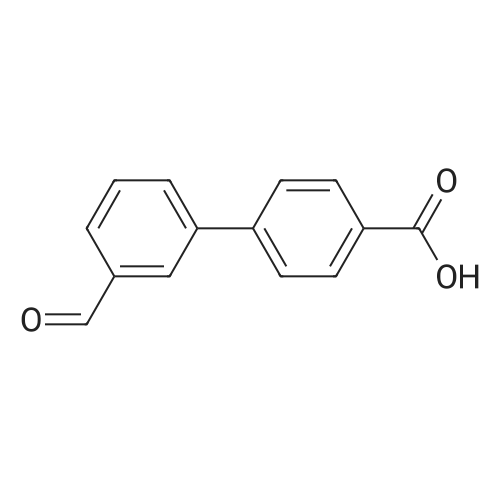
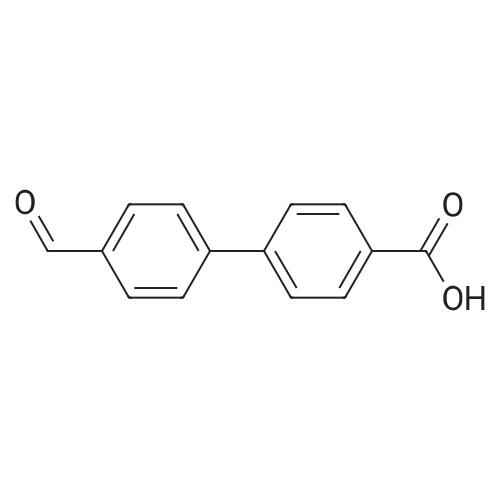
|
With thionyl chloride; for 2h;Reflux; |
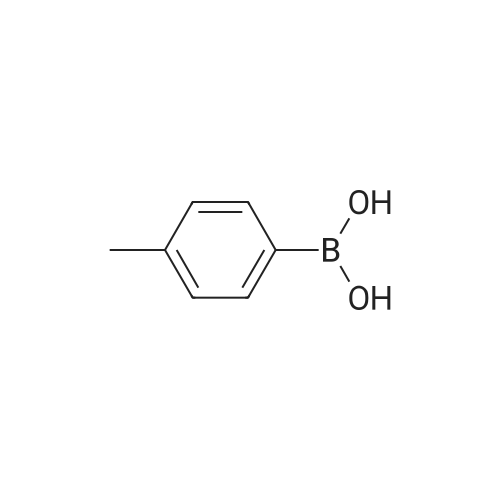
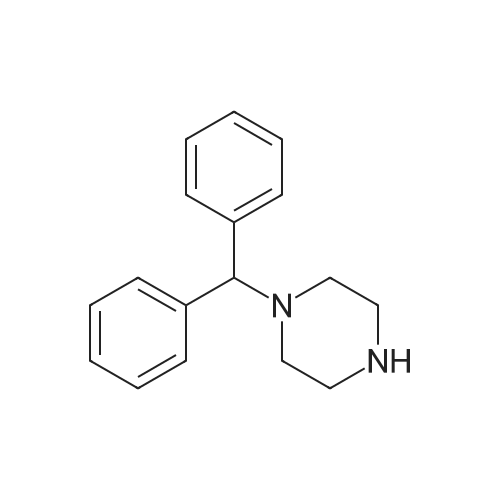
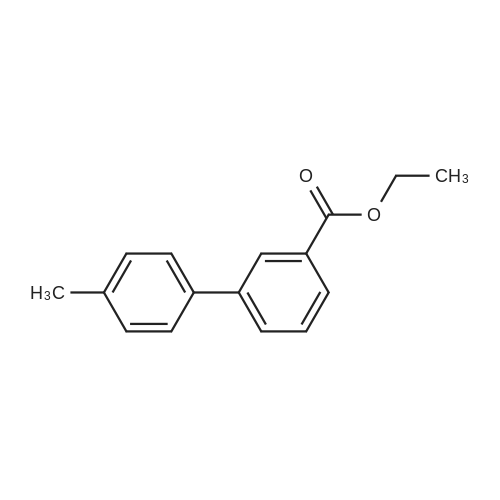
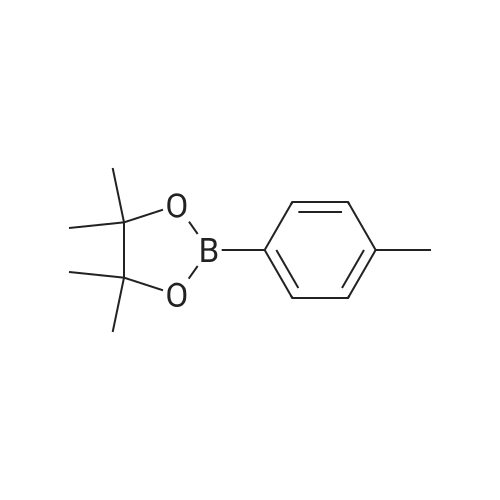
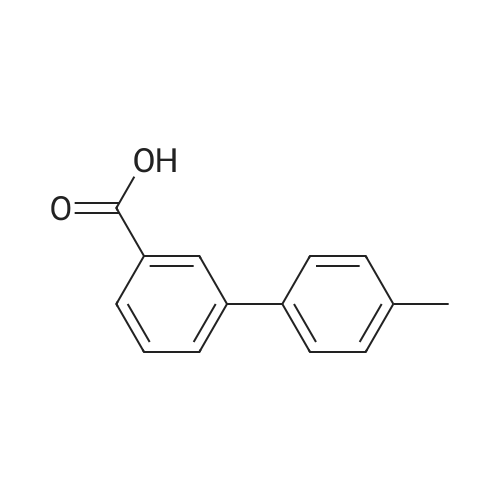
Ethyl 3-bromobenzoate (2.43 g) was mixed with p-methylphenylboronic acid (1.50 g), 2.12 g of sodium carbonate, 2.31 g of tetrakis(triphenylphosphine)palladium and 30 mL of water were added, and the mixture was stirred at reflux under nitrogen and stirred overnight. After cooling, the solution was evaporated by evaporation and the mixture was separated on a silica gel column (isolation system: petroleum ether:ethyl acetate = 10:1 to 5:1) to obtain 1.61 g of a coupling product in a yield of 71%. 2.26 g of the product in the previous step was added to a mixed solution of 20 mL of tetrahydrofuran and 5 mL of methanol. 100 mL of a 20% aqueous lithium hydroxide solution was added to the solution, and the mixture was stirred at room temperature overnight. After the completion of the process, the pH is adjusted to 2 with concentrated hydrochloric acid and the solvent is distilled off. The mixture was separated on a silica gel column (isolation system: petroleum ether:ethyl acetate=10:1 to 100% ethyl acetate) to obtain 1.59 g of a hydrolyzed product with a yield of 75%. The product of the previous step was directly added with 50 mL of thionyl chloride and stirred at reflux for 2 h. Thionyl chloride was distilled off to obtain the product of the acid chloride, which was used directly in the next step. 3.46 g of the acid chloride product and 2.29 g of the intermediate compound were mixed and 50 mL of pyridine was added. 100C, stirred at reflux overnight. After cooling, the solvent was distilled off and the mixture was separated on a silica gel column (isolation system: petroleum ether:ethyl acetate=10:1 to 100% ethyl acetate) to give a crude product. The crude product was purified by preparative chromatography (mobile phase: water (0.05% aqueous ammonia)-acetonitrile; B 40%-80%, 50 minutes) to give a pale orange solid 0.85 g, yield 20%. |
|
With pyridine; thionyl chloride; In toluene; |
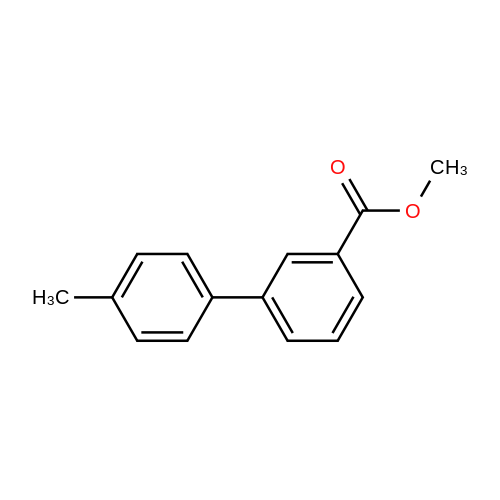
4'-Methyl-biphenyl-3-carboxylic acid (300 mg, 1.4 mmol) was added to a solution of thionylchloride (0.13 ml, 1.8 mmol) in toluene (3 ml) at O0C. After 2 min, pyridine (2 drops) was added, and stirring was continued overnight. After removal of the solvent and addition of dichloromethane (1.5 ml), 4-amino-l-hydroxymethyladamantane was added together with DIPEA (0.31 ml, 1.8 mmol). The mixture was stirred overnight and purified by preparative LC-MS to give the title compound as a mixture of two isomers. 1H NMR (400 MHz, CDCI3) δ:7.94 - 7.99 (m, 1 H), 7.65 - 7.74 (m, 2 H), 7.46 - 7.54 (m, 3 H), 7.25 - 7.30 (m, 2 H), 6.38 - 6.49 (m, 1 H), 4.15 - 4.28 (m, 1 H), 3.22 - 3.29 (m, 2 H), 2.41 (s, 3 H), 2.13 - 2.22 (m, 2 H), 2.00 - 2.06 (m, 1 H), 1.42 - 1.95 (m, 10 H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping