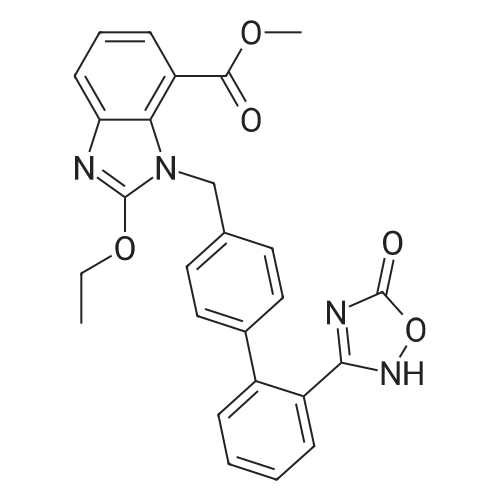
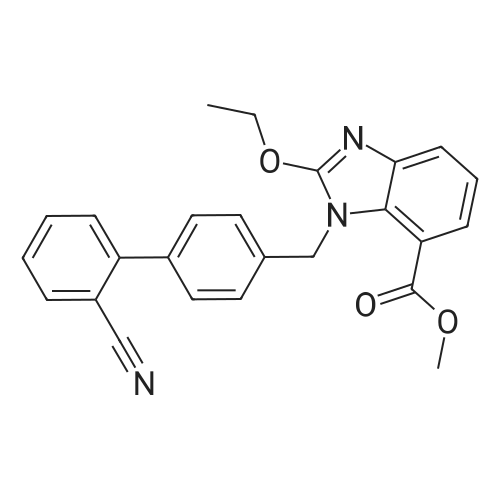
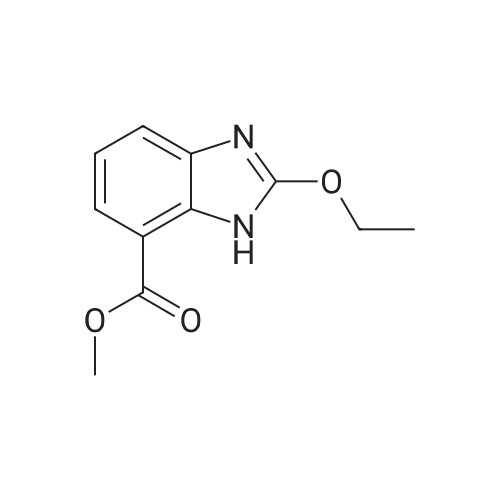
| 95.6% |
With water; sodium hydroxide; at 20 - 30℃; for 2h; |
45 g (0.1 mol) of compound 2 (methyl ester) were dissolved in 450 ml of dimethylsulfoxide and separately controlled with carbon dioxideSpeed 80ml / min and flow rate 1.2L / min into the microreactor, after the temperature control unit in the reaction unit at 90-100 , PaulHold 1.0MPa pressure reaction for 60 seconds. The microreactor was obtained Azithromycin methyl ester solution by gas-liquid separator, adding 1mol / L hydrogenSodium hydroxide solution 450ml, the reaction temperature 20-30 ° C under stirring 2h, adding toluene 450ml, with hydrochloric acid to adjust the pH = 3-5,Liquid separation, after removal of toluene by adding ethanol 400ml, dissolved after cooling crystallization, and dried to give a white solid 43.6g, yield95.6percent, HPLC purity greater than 99.8percent. |
| 89.1% |
With methanol; sodium hydroxide;Reflux; |
A mixture of 65 g of the intermediate <strong>[147403-52-9]2-ethoxy-1-((2'-(2,5-dihydro-5-oxo-1,2,4-oxadiazol-3-yl)biphenyl-4-yl)methyl)benzimidazole-7-carboxylic acid methyl ester</strong>, And 620 ml of methanol were charged into a reaction flask, turn on agitation,Then, 160 g of 10percent sodium hydroxide solution was added,Rose to reflux insulation reaction,Controlled by HPLC.After completion of the reaction, the temperature was lowered to 15-25 ° C,18 g of activated carbon was added,Stirring bleaching for half an hour.Filtered, washed with 30 ml of purified water,Filtration to dry, dropping hydrochloric acid,Adjust the pH to 2 to 3, adjust the mixing 1h, filter, with 30ml of purified water washing, filter to dry to get crude. The crude product was added to 200 ml of methanol, stirring, heating reflux 1h, cooling to 0 ~ 5 , stirring 1h, suction filtration,Washed with 20 ml of methanol, suction filtered to dryness,At 60 ~ 70 ° C to dry to dry azithentin into 56.1 g, yield 89.1percent, purity 99.6percent. |
| 88.7% |
With methanol; sodium hydroxide; at 20℃; for 10h; |
Into a 500 ml round bottom flask, 10 g of a raw material A4, 110 ml of methanol was added, and 28.8 ml of a 10percent NaOH solution was added under stirring at room temperature, and the reaction was stirred for 10 hours. After the reaction was completed, the methanol was spun dry. And adding 200 ml of water to the system, and adjusting the pH to 3-4 with HCl (1 mol/L), At this point a large amount of white solids are produced, suction filtration, Obtain crude product, separate and purify by column chromatography. Dichloromethane: methanol (1:20) gave 8.6 g of a white solid, yield 88.7percent, purity 97.4percent. |
| 88% |
With pyrographite; potassium hydroxide; In water; at 50℃; for 1h; |
20 g of compound II-1 was added to a 5percent aqueous KOH solution.Stir the mixture to 50 ° C for 1 h, add activated carbon and continue to stir.Filter and collect the filtrate. Cool down to 10 ° C, adjust pH to 1 with hydrochloric acidFiltration, washing and drying to obtain Compound I, the yield is 88percent |
| 77.4% |
With sodium hydroxide; at 73 - 75℃; for 2h; |
Compound (5A) 2.0 g, placed in a reaction flask, 0.4mol / L sodium hydroxide, 32ml, 73-75 reaction for 2 hours.After the reaction was cooled to room temperature, a solution of aqueous acid solution to pH = 3-4, the precipitated white solid 1.5g, 77.4percent. |
| 75% |
|
Example 3Preparation of l-[[2'-(4, 5-dihydro-5-oxo-4H-l, 2, 4-oxadiazol-3-yl) biphenyl-4-yl] methyl]-2-ethoxy-lH-benzimidazoIe-7-carboxylic Acid (Azilsartan)Methyl l-[[2'-(4,5-dihydro-5-oxo-4H-l,2,4-oxadiazol-3-yl) biphenyl-4-yl] methyl]-2-ethoxy-lH-benzimidazole-7-carboxylate (30 g) and NaOH solution (0.4 N; 475 ml) was stirred at 50-55°C for 30 min. Reaction mass was cool to 10-15°C and water was added. The pH of the resulting solution was adjusted to 2-3 by using 2 N HC1. Reaction mass was stirred for 30 mins. at 20-25°C. The product was filtered and dried under vacuum. The resulting product was suspended in isopropyl alcohol and was stirred for 25-30 mins. at 40-45°C. The product was filtered, washed and dried to obtain title compound. (Yield: 22 g; 75percent) |
|
|
Example 11 2-Ethoxy- 1 -((2'-(5-oxo-4,5-dihydro- 1 ,2,4-oxadiazol-3-yl)biphenyl-4-yl)methyl)- 1 H- benzo[c/]imidazole-7-carboxylic acid - <strong>[147403-52-9]azilsartan</strong> of formula IIA mixture of ethyl 2-ethoxy-l-((2'-(5-oxo-4,5-dihydro-l ,2,4-oxadiazol-3-yl)biphenyl-4- yl)methyl)-l H-benzo[< ]imidazole-7-carboxylate (of formula la; 3.2 g, 6.6 mmol) and aqueous sodium hydroxide (0.8 g of NaOH in 50 ml of water) was stirred at the temperature of 70°C for 1.5 hours. After cooling to the room temperature the mixture was acidified with 10percent HCl until pH 3 while being stirred and cooled. The insolubles were aspirated and washed with water. 2.6 g (86.2 percent) of a product was obtained containing 97.2 percent of <strong>[147403-52-9]azilsartan</strong> of formula II with the melting point of 208 to 21 1 °C according to HPLC. NMR spectrum: NMR (500 MHz, DMSO) delta (ppm): 13.17 (bs, 1H, OH or NH), 12.42 (bs, 1H, OH or NH), 7.70-7.60 (m, 3H, Ar), 7.57-7.50 (m, 2H, Ar), 7.50-7.44 (m, 1 H, Ar), 7.23 (d, J = 8.3 Hz, 2H, Ar), 7.18 (t, J = 7.9 Hz, lH, Ar), 7.05 (d, J = 8.3 Hz, 2H, Ar), 5.68 (s, 2H, N-CH2-Ar), 4.58 (q, J = 7.1 Hz, 2H, OCH2CH3), 1.38 (t, J = 7.1 Hz, 3H, OCH2CH3). |
|
With lithium hydroxide; In water; at 20℃; for 16h; |
10 g of the <strong>[147403-52-9]azilsartan</strong> intermediate compound I crystal obtained in this example dispersed in 45 mL of water. Then, 5.0 mL of lithium hydroxide aqueous solution with a solute mass fraction of 20percent was added. After stirring at room temperature for 16 hours, the aqueous solution was acidified with dilute hydrochloric acid to rhoH- = 3. Filtration and ethanol recrystallization gave <strong>[147403-52-9]azilsartan</strong> product with a purity of 99percent. |
| 82.5 g |
With lithium hydroxide; In methanol; water; for 3h;Reflux; |
placed <strong>[147403-52-9]<strong>[147403-52-9]azilsartan</strong> methyl ester</strong> 100 g, methanol 730mL to 5000mL four-neck flask equipped with two stirring blades with a diameter of 15cm, was dissolved by heating with stirring.Was added thereto 2N aqueous lithium 590mL hydroxide, was heated to reflux temperature, the reaction was conducted for 3 hours.The resulting reaction solution was cooled to room temperature to prepare a pH of the reaction solution with 2N aqueous hydrochloric acid solution 3.And concentrating the reaction solution, the resulting residue in water 1200 mL, stirred with dichloromethane 3000 mL 30 minutes, allowed to stand for 15 minutes, was fractionated and the dichloromethane layer by a liquid.The resulting dichloromethane solution was concentrated to the resulting residue was stirred overnight at 20 to 30 ° C. was added ethyl acetate 2000 mL.Then, a sample was collected under reduced pressure filtered and precipitated crystals were dried at 50 ° C., to give colorless prisms of <strong>[147403-52-9]azilsartan</strong> of 82.5 g (<strong>[147403-52-9]azilsartan</strong> purity: 96.12percent). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping